Figures & data
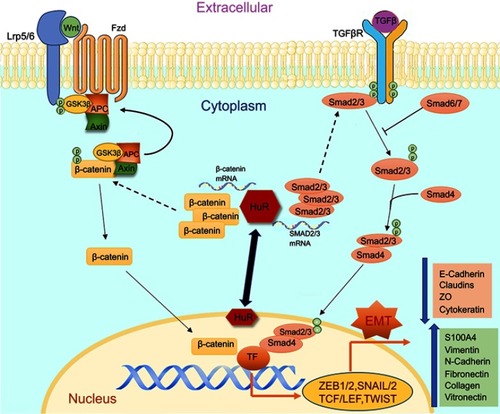
Figure 1 The figure illustrates the cellular pathways associated with EMT and HuR as a modulator regulating the changes in smokers and COPD. Both TGFβ and Wnt pathways play a crucial role in inducing EMT and potentially activate their associated receptors TGFβR and Frizzled, respectively. Activation of TGFβ-TGFβR results in phosphorylation of SMAD2/3 which tags with SMAD4 and translocates into the nucleus causing transcriptional changes. Activation of Wnt-Frizzled receptor signaling leads to inhibition of GSK3β and consequential accumulation of cytoplasmic β-catenin. The free β-catenin translocates into the nucleus and regulates target gene transcription. Finally, both pathways could lead to the induction of EMT with increases in mesenchymal and corresponding decreases in epithelial proteins. HuR, under pathological conditions, translocates from peri-nuclear into the cytoplasm and enhances further synthesis and stabilization of these translocating nuclear proteins, thus increasing the potential of EMT.