Figures & data
Table 1 Patient Demographics and Baseline Characteristics
Table 2 Lung Function Endpoints
Figure 2 Change from baseline in (A) trough FEV1 and (B) trough FVC.
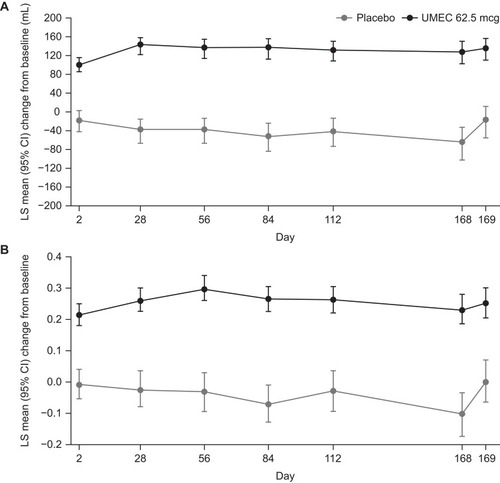
Figure 3 TDI focal score at Days 28, 84, and 168.
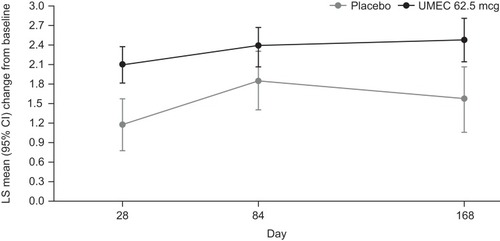
Table 3 Proportion of TDI Responders (Defined as a TDI Focal Score ≥1 Unit)
Table 4 Rescue Medication Use, Mean SGRQ Total Score and CAT Score
Table 5 Summary of on-Treatment AEs