Figures & data
Table 1 Patient Demographics And Clinical Characteristics Overall And For Each Country
Table 2 Demographics And Clinical Characteristics Of Patients Stratified By Blood Eosinophil Counts
Table 3 Demographics And Clinical Characteristics Of Patients With COPD By Exacerbations History/Current Therapy
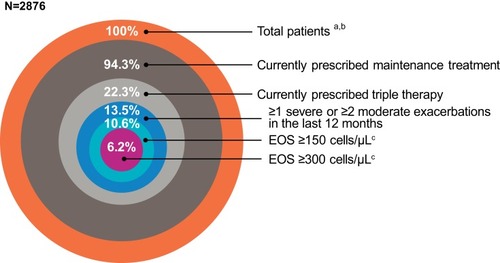
Figure 1 Proportion of patients from the total diagnosed COPD sample (weighted absolute percentages) who were prescribed triple therapy, had ≥2 moderate or ≥1 severe AECOPD, and had a blood eosinophil count ≥150 cells/µL and ≥300 cells/µL in the five European countries studied.
Abbreviations: AECOPD, acute exacerbations of COPD; COPD, chronic obstructive pulmonary disease; EOS, blood eosinophil count.
Data Availability
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies which evaluate medicines, upon approval of proposals submitted to www.clinicalstudydatarequest.com. To access data for other types of GSK sponsored research, for study documents without patient-level data and for clinical studies not listed, please submit an enquiry via the website. The data that support the findings of this study are available from Adelphi Real World where restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of Adelphi Real World.
