Figures & data
Table 1 Demographic and Baseline Disease Characteristics (Japanese Safety Population)
Figure 1 Patient disposition.
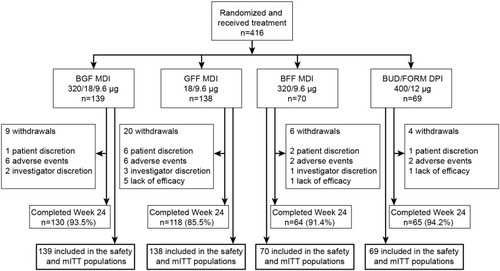
Table 2 Primary and Secondary Lung Function Endpoints (Efficacy Estimand; mITT Population)
Figure 2 Change from baseline in morning pre-dose trough FEV1 over 24 weeks (efficacy estimand; Japanese mITT population).
Abbreviations: BFF, budesonide/formoterol fumarate; BGF, budesonide/glycopyrrolate/formoterol fumarate; BUD/FORM DPI, budesonide/formoterol fumarate dry powder inhaler; FEV1, forced expiratory volume in 1 s; GFF, glycopyrrolate/formoterol fumarate; LSM, least squares mean; MDI, metered dose inhaler; mITT, modified intent-to-treat.
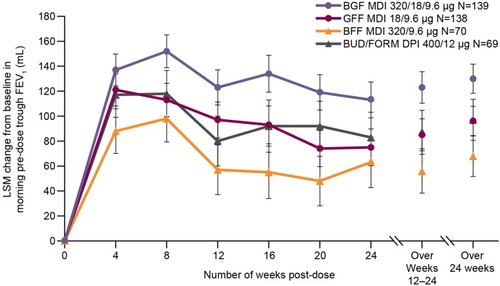
Table 3 Symptom and Quality of Life Endpoints (Efficacy Estimand; mITT Population)
Table 4 CIDa and COPD Exacerbations (Efficacy Estimand; Japanese mITT Population)
Table 5 Summary of Adverse Events (Japanese Safety Population)
