Figures & data
Table 1 Patient Demographics And Baseline Characteristics In The Japan Subgroup And Overall Study Population (ITT population)
Figure 1 On-treatment moderate/severe exacerbations in the Japan subgroup and overall study population with FF/UMEC/VI versus dual therapies (ITT populations).
Abbreviations: FF, fluticasone furoate; ITT, intent-to-treat; UMEC, umeclidinium; VI, vilanterol.
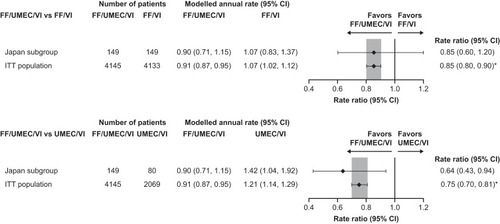
Figure 2 Time-to-first on-treatment moderate/severe exacerbation in the Japan subgroup and overall study population with FF/UMEC/VI versus dual therapies (ITT populations).
Abbreviations: FF, fluticasone furoate; ITT, intent-to-treat; UMEC, umeclidinium; VI, vilanterol.
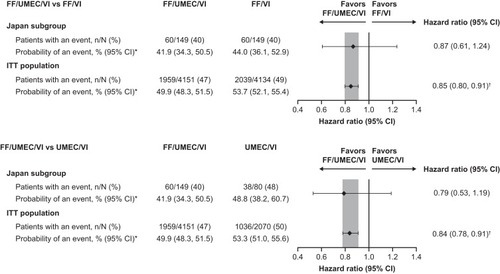
Figure 3 Change from baseline at Week 52 in (A) trough FEV1 and (B) post-bronchodilator FEV1 in the Japan subgroup and overall study population with FF/UMEC/VI versus dual therapies (ITT populations).
Abbreviations: FF, fluticasone furoate; ITT, intent-to-treat; LS, least squares; UMEC, umeclidinium; VI, vilanterol.
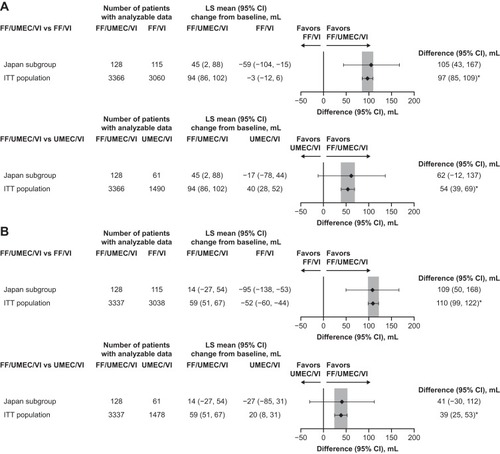
Figure 4 Change from baseline at Week 52 in (A) SGRQ total score and (B) CAT score in the Japan subgroup and overall study population with FF/UMEC/VI versus dual therapies (ITT populations).
Abbreviations: CAT, Chronic obstructive pulmonary disease Assessment Test; FF, fluticasone furoate; ITT, intention to treat; LS, least squares; SGRQ, St. George’s Respiratory Questionnaire; UMEC, umeclidinium; VI, vilanterol.
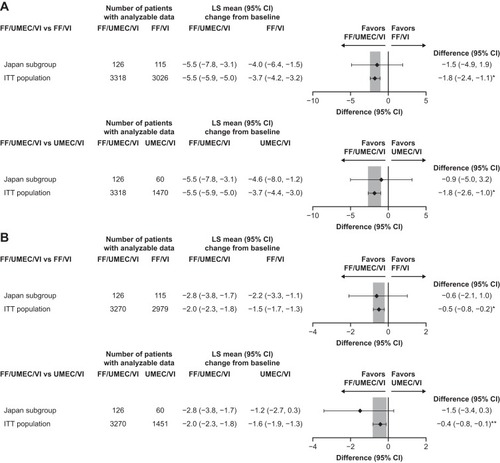
Table 2 Safety Summary In Japan Subgroup And Overall Study Population (ITT populations)
Data Availability
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
