Figures & data
Figure 1 Reduced GRα expression and activity in COPD HBE cells. (A) GRα expression in DHBE (also described as COPD HBE in the text) and NHBE cells, determined by Western blotting and quantified by densitometric analysis. β-Actin served as a loading control. (B) DNA-binding activities of GRα and NF-κB p65 in NHBE and DHBE cells. Data are expressed as means ± SD. n = 4; ***P < 0.001.
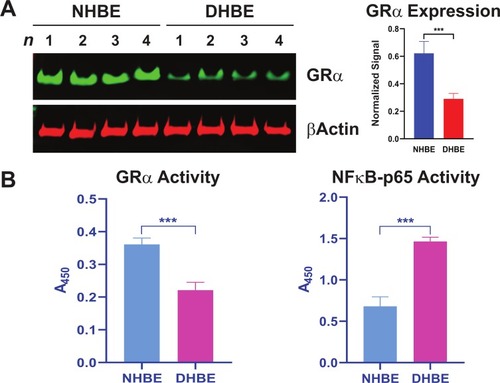
Figure 2 Rofl induces GRα expression in COPD HBE cells. (A-B) COPD HBE cells were treated with the indicated concentrations of Rofl for 6 h (A) or with 1 µM of Rofl for the indicated periods (B) and GRα levels determined by Western blotting in whole-cell extracts and quantified by densitometric analysis. β-Actin served as a loading control. (C) Time course of transcriptional response to Rofl. After COPD HBE cells were treated with 1 µM Rofl or Veh control for 6 h, nascent mRNA captured by Click-iT Nascent RNA Capture Kit. Relative mRNA levels of GRα were measured by real-time PCR. (D) Effects of Rofl vs. Veh control on GRα mRNA stability in COPD HBE cells were determined by incubation in growth medium containing 5-EU followed by incubation in growth medium without 5-EU for the indicated periods. After total mRNA isolation, labeled mRNA was captured and analyzed with Click-iT Nascent RNA Capture Kit. Data are expressed as means ± SD; n = 3. **P < 0.01, ***P < 0.001.
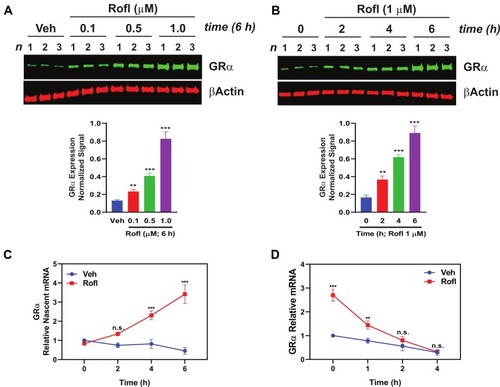
Figure 3 Rofl stimulates GRα promoter activity and induces GRα transcriptional activity. (A) Promoter activity. COPD HBE cells were treated with Rofl (0.5 or 1 µM) or Veh for 6 h, and then nuclear extracts were obtained and incubated with the indicated biotinylated double-stranded oligonucleotides corresponding to the following: WT or Mu GRα-CRE, the consensus CRE (positive control), or a nonspecific sequence (negative control). Bead-bound oligonucleotide-protein complexes were eluted and subjected to Western blotting to identify the presence of CREB. Western blotting for Lamin B1 was used as a control for non-specific interaction. Nuclear extracts without added nucleotides were loaded as input. (B) Transcriptional activity. GRα reporter cells were treated with the indicated concentrations (0–3 nM) of Rofl, Dex, or a combination of both for 24 h. GRα transcriptional activity, shown as the percent maximal response, was then measured using a GRα-specific reporter assay. Concentration-response curve fitting was performed by non-linear regression. Results were reproduced independently at least twice. Data are expressed as means ± SD; n = 3, ***P < 0.001.
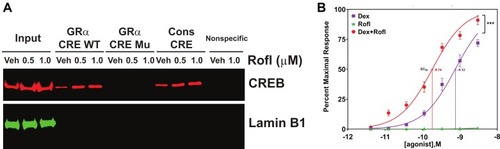
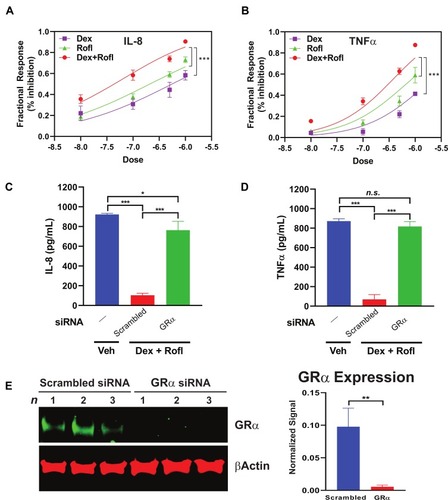
Figure 4 Rofl inhibits IL-8 and TNFα production in COPD HBE cells additively with Dex in a GRα-dependent manner. (A-B) COPD HBE cells were treated with Rofl, Dex, or combined Rofl and Dex at indicated concentrations for 6 h. Culture medium was then collected, and IL-8 (A) and TNFα (B) levels in medium were determined by ELISA. Inhibitory effects of treatments are shown as fractional response. Curves were fitted by non-linear regression and the Bliss independence model. (C-D) COPD HBE cells received 24 h-transfection with scrambled or GRα siRNA and then were treated with Veh or combined Rofl and Dex (1 µM each) for 6 h. IL-8 (C) and TNFα (D) levels in culture medium were determined by ELISA. Data are expressed as means ± SD; n = 3. *P < 0.05, ***P < 0.001. (E) GRα expression in siRNA-transfected COPD HBE cells was determined by Western blotting and quantified by densitometric analysis. β-Actin served as a loading control. **P < 0.01.