Figures & data
Table 1 Baseline Characteristics
Figure 1 Study flow. The decrease in the number of participants has several reasons: the cohort-study was initially designed as a cross-sectional trial. During the course of the first year, the design was changed to a longitudinal study intended to analyse the course of lung function, exacerbations, cardiovascular outcomes, and further parameters over a period of 3 years. The participants gave consent to only one study visit initially and, when asked again after the first year, a great number of participants did not agree to taking part in a 3-year-study. Then again, after 3 years, the study period was extended and only a part of the participants agreed to continue. Additionally, parts of the initial COPD-cohort had died within the study period.
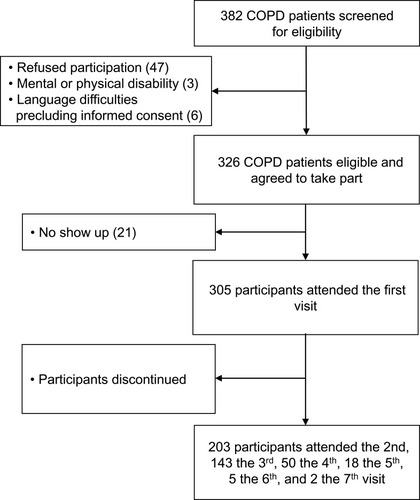
Table 2 Overview on Medication of the Cohort, and Accordance with 2006 GOLD Guideline (Visits That Took Place Before 2011)
Table 3 Overview on Medication of the Cohort, and Accordance with GOLD 2011/2014 (Visits from 2011 on)
Figure 2 Distribution of prescribed medication and accordance with guidelines. (A) By risk-category: distribution of prescribed medicine. Nineteen percent of participants were categorised group A, 24.8% B, 8.6% C, and 47.6% D. Visits, in which the patient had no medication were not depicted in (A) due to clarity reasons. (B) By GOLD-stage (visits before 2011), or risk-category (visits from 2011 on): amount of visits where correct treatment, undertreatment, and overtreatment was observed. In visits before 2011, 12% of patients were GOLD-stage 1, 26% stage 2, 41% stage 3, and 21% stage 4. In visits from 2011 on, 19% of participants were categorised group A, 24.8% B, 8.6% C, and 47.6% D.
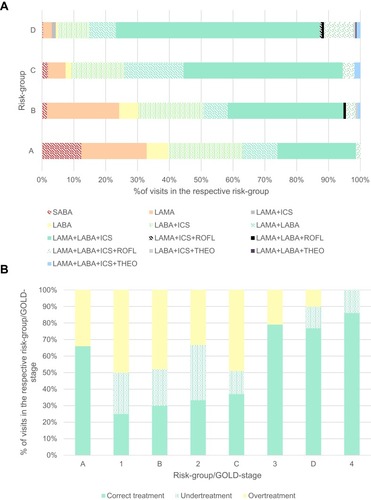
Table 4 Comparison of Clinical Characteristics Between Groups
