Figures & data
Table 1 Definitions of ECII
Table 2 Baseline Demographics and Clinical Characteristics (Full Analysis Set)
Figure 1 Proportion of patients achieving ECII at Weeks 4 or 12. ECII definition 1: improvement in trough FEV1 ≥100 mL and reduction in SGRQ-C total score ≥4; ECII definition 2: improvement in trough FEV1 ≥100 mL and reduction in CAT score ≥2; n, number of patients who achieved ECII. N, number of patients who had Day 1 and post-baseline values (either at Week 4 or 12) corresponding to parameters used to evaluate ECII by the two definitions.
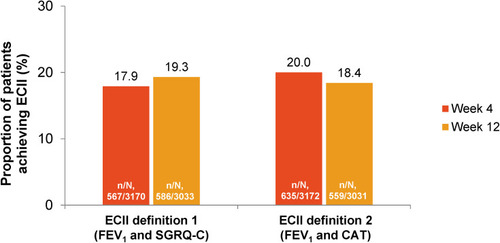
Figure 2 Annualized rate of moderate or severe exacerbations by ECII definition at Weeks 4 and 12. The exacerbation rates were assessed from Week 4 to 52 or Week 12 to 52 in patients who achieved ECII at Week 4 or 12, respectively. ECII definition 1: improvement in trough FEV1 ≥100 mL and reduction in SGRQ-C total score ≥4; ECII definition 2: improvement in trough FEV1 ≥100 mL and reduction in CAT total score ≥2; n, number of patients included in this analysis. Moderate or severe COPD exacerbations starting from Week 4 or 12 and one day after date of last treatment are included.
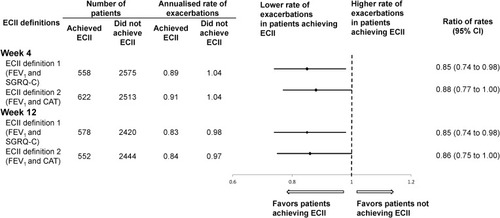
Figure 3 Time-to-first moderate or severe exacerbation from (A) Week 4; and (B) Week 12 to the end of treatment by ECII definitions. The time-to-first exacerbation was assessed from Week 4 to 52 or Week 12 to 52 in patients who achieved ECII at Week 4 or 12, respectively. ECII definition 1: improvement in trough FEV1 ≥100 mL and reduction in SGRQ-C total score ≥4; ECII definition 2: improvement in trough FEV1 ≥100 mL and reduction in CAT score ≥2. Moderate or severe COPD exacerbations starting from Week 4 or 12 and one day after date of last treatment are included.
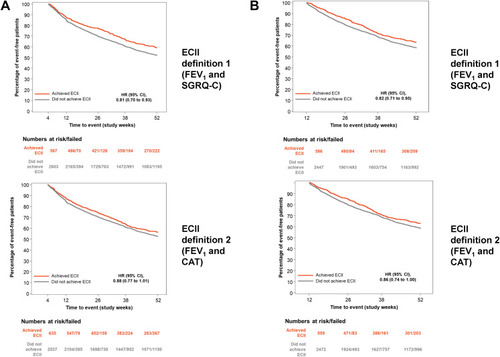
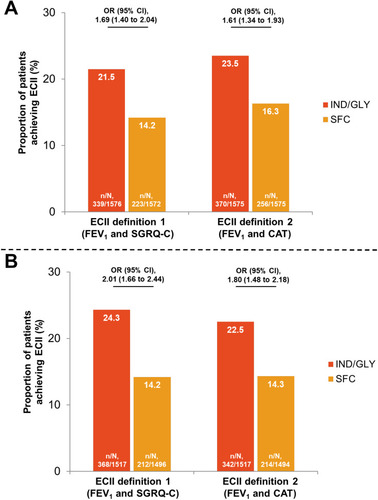
Figure 4 Proportion of patients achieving ECII with IND/GLY and SFC at (A) Week 4; and (B) Week 12. ECII definition 1: improvement in trough FEV1 ≥100 mL and reduction in SGRQ-C total score ≥4; ECII definition 2: improvement in trough FEV1 ≥100 mL and reduction in CAT total score ≥2; n, number of patients who achieved ECII at Week 4 or 12; N, number of patients corresponding to the respective treatment group included in the analysis.