Figures & data
Figure 1 Study flow chart.
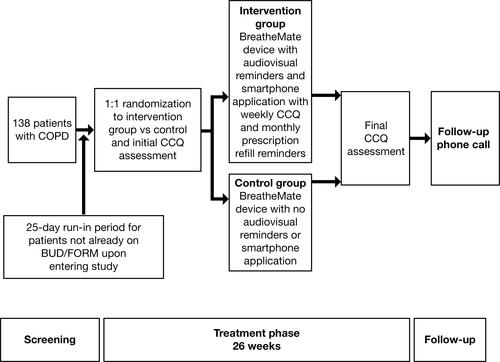
Figure 2 BreatheMate, smartphone application, and example patient data.
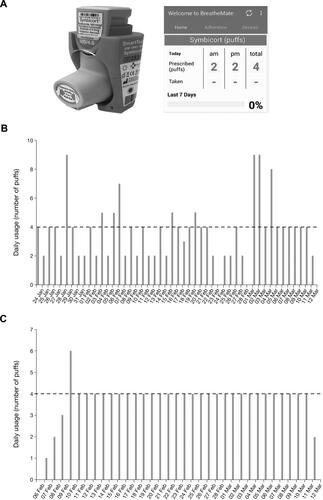
Table 1 Patient Demographics
Figure 3 CONSORT flow diagram.
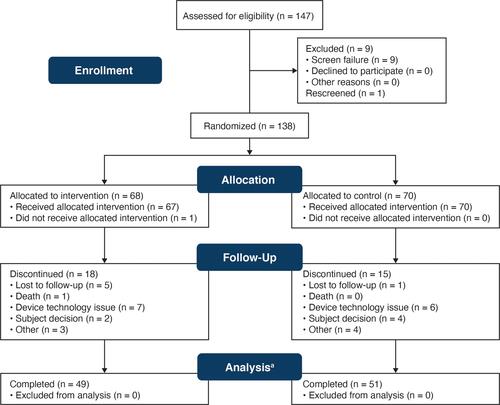
Figure 4 Mean adherence to budesonide/formoterol during the study: FAS.
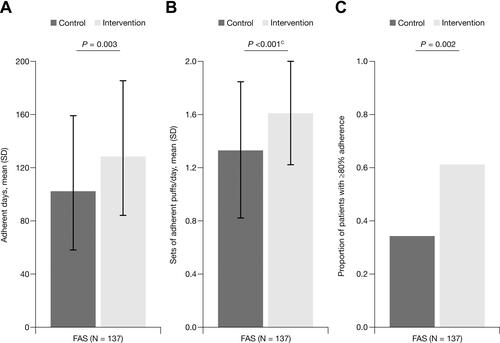
Figure 5 Mean sets of adherent budesonide/formoterol puffs/day during each 60-day study interval: FAS.
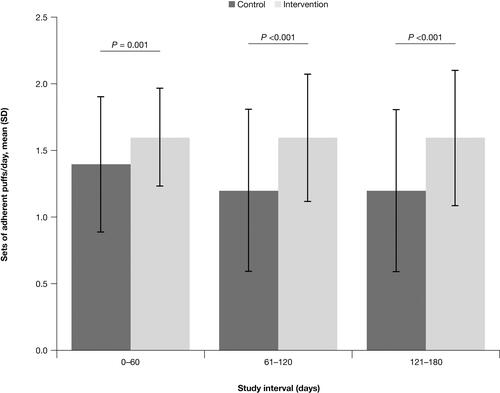
Figure 6 Number of adherent,a underuse,b overuse,c and no used days: FAS.
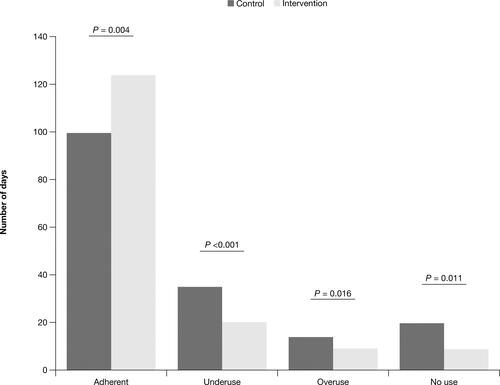
Figure 7 Odds of ≥80% adherence by subgroup: FAS (95% CI).a
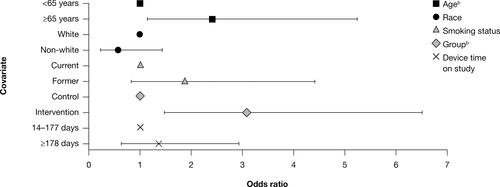
Figure 8 Mean CCQ scores at baselinea and EOTb: FAS.
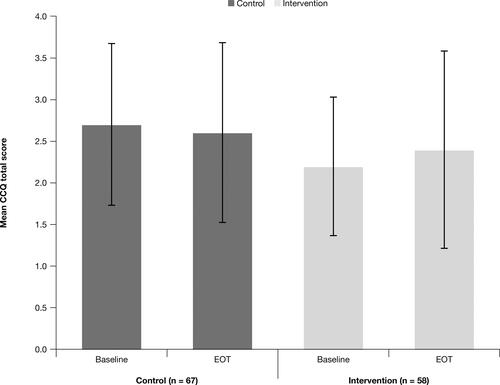
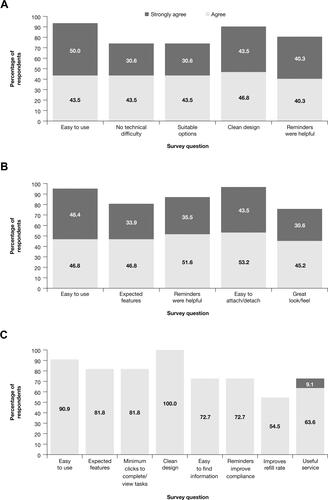
Figure 9 Patient and provider satisfaction reports.