Figures & data
Figure 1 Flow diagram of the patient selection process for post hoc analysis.
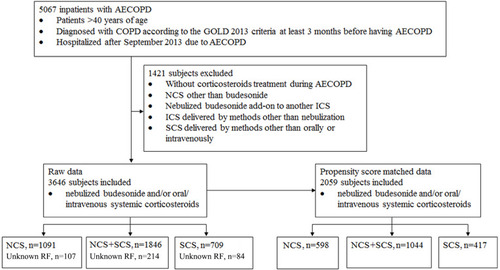
Table 1 Baseline Characteristics of Hospitalized AECOPD Patients Treated with NCS, SCS, or NCS+SCS Before Propensity Score Matching
Table 2 Clinical Outcomes of Hospitalized AECOPD Patients Treated with NCS, SCS, or NCS+SCS Before Propensity Score Matching
Figure 2 Frequency and logistic regression results for categorical data of clinical outcomes. NCS was used as the reference; adjusted odds ratio was adjusted by age, sex, height, weight, duration of COPD, duration of initial AECOPD, PaO2 at the first time, PaCO2 at the first time, SaO2 at the first time and pH at the first time. OR values of some clinical outcomes were very large or small, and the exact value could not be obtained, *was used instead.
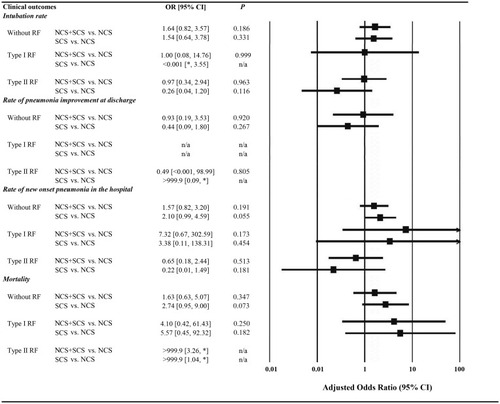
Figure 3 The final general linear model results for continuous data of clinical outcomes. NCS was used as the reference; the adjusted regression coefficient was adjusted by age, sex, height, weight, duration of COPD, duration of initial AECOPD, PaO2 at the first time, PaCO2 at the first time, SaO2 at the first time and PH at the first time. The value greater than 8 or less than −8 was not shown in the graph on the right.
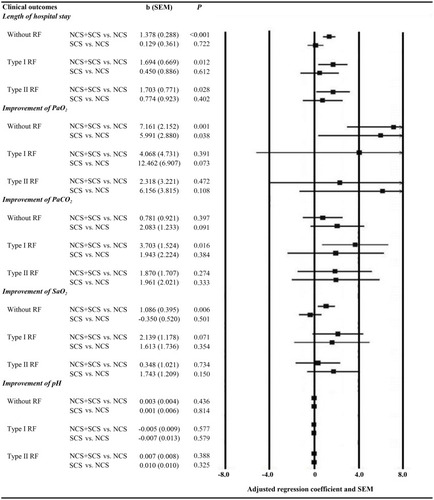
Table 3 Clinical Outcomes of Hospitalized AECOPD Patients without RF After Propensity Score Matching
Table 4 Clinical Outcomes of Hospitalized AECOPD Cases with RF After Propensity Score Matching
