Figures & data
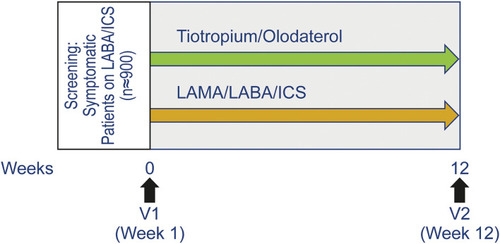
Figure 1 GOLD 2020 follow-up pharmacologic treatment recommendations.*Consider if eos ≥ 300 or eos ≥ 100 AND ≥ 2 moderate exacerbations/1 hospitalization. **Consider de-escalation of ICS or switch if pneumonia, inappropriate original indication or lack of response to ICS. ©2020, Global Initiative for Chronic Obstructive Lung Disease, reproduced with permission.Citation1
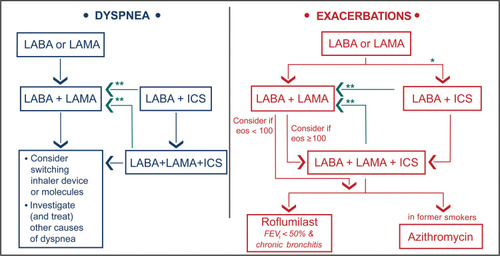
Figure 2 EVELUT® study overview.