Figures & data
Figure 1 Study design. aFour waves of data collection were required to achieve the target sample size. The dates of the baseline period were: June 1, 2017 to May 31, 2018 (Wave 1); July 1, 2017 to June 30, 2018 (Wave 2); August 1, 2017 to July 31, 2018 (Wave 3); and October 1, 2017 to September 30, 2018 (Wave 4). Due to the claims lag, fully adjudicated medical and pharmacy claims data for each wave were available approximately 6 months after the end of the baseline period.
Figure 2 Study attrition. Percentages may not sum to 100% due to rounding. aCOPD ICD-10 diagnosis codes were J40, J410, J411, J418, J42, J430, J431, J432, J438, J439, J440, J441, and J449; btriple therapy is defined as claims for ICS/LABA/LAMA, including combined monotherapy formulations and fixed-dose combinations. Patients with no evidence of triple therapy within 30 days were included; casthma ICD-10 diagnosis codes were J4520, J4521, J4522, J4530, J4531, J4532, J4540, J4541, J4542, J4550, J4551, J4552, J45901, J45902, J45909, J45990, J45991, and J45998; dfor waves in which a larger sampling frame was available than was needed to meet the current target, random sampling was implemented to derive the survey sampling frame. For Waves 2–4, patients were excluded if they had been invited to participate in the survey in a previous wave; efollowing a 6-month claims lag, patients were removed from the analysis if they had evidence of asthma, lung cancer (diagnosis or treatment) or if they had evidence of triple therapy during the 12-month baseline period. Continuous enrollment in the health plan was also re-assessed, and patients with <12 months of continuous enrollment in the baseline period were excluded. Patients that self-reported a medication that was different from the claims-based medication cohort were also excluded from the analysis (n=29).
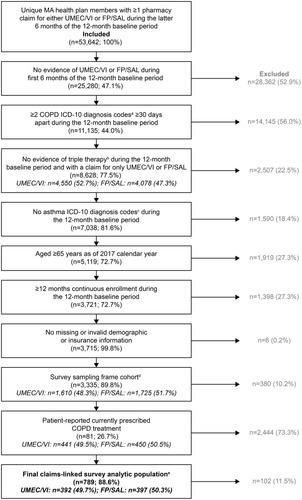
Table 1 Patient Demographics by Treatment Cohort
Figure 3 Mean CAT scores among patients receiving UMEC/VI or FP/SAL before IPTW.
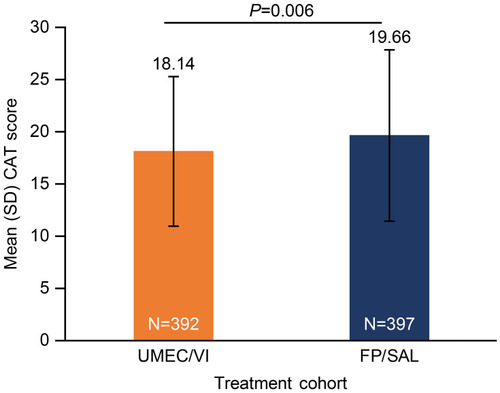
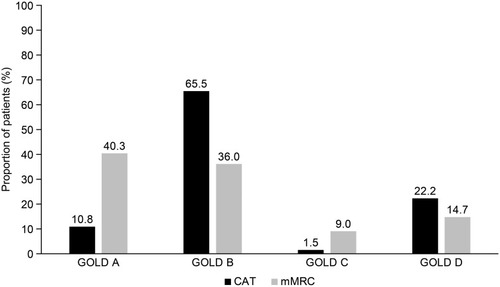
Figure 4 GOLD classification according to symptom burden (assessed by CAT and mMRC) and exacerbation history.
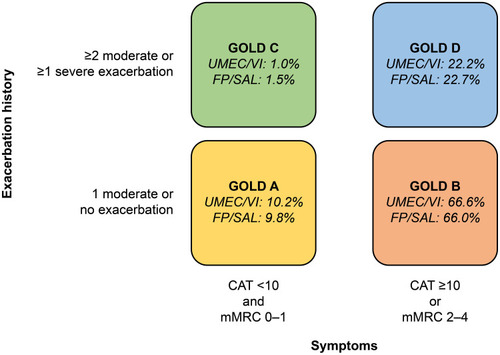
Figure 5 Comparison of GOLD classification according to different patient-reported measures of symptom burden (CAT or mMRC).