Figures & data
Figure 1 GOLDEN 3 and GOLDEN 4 study design.
Abbreviations: CV, cardiovascular; GOLDEN, Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer; LABA, long-acting beta agonist.
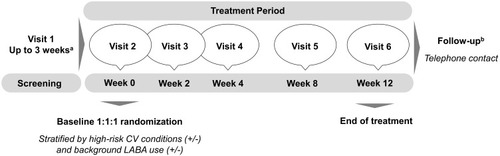
Table 1 Baseline Demographic and Clinical Characteristics of the Pooled GOLDEN 3 and GOLDEN 4 ITT Study Cohorts
Table 2 Proportions of Subjects Meeting Criteria for CID at Treatment Week 12
Figure 2 Risk (adjusted odds ratio)a of CID at week 12 of treatment with GLY (25 mcg BID and 50 mcg BID) compared to placebo.
Abbreviations: BID, twice daily; CI, confidence interval; CID, clinically important deterioration; FEV1, forced expiratory volume in one second; GLY, glycopyrrolate; HCRU, healthcare resource utilization; LABA, long-acting beta-agonist; OR, odds ratio; SGRQ, St. George’s Respiratory Questionnaire.
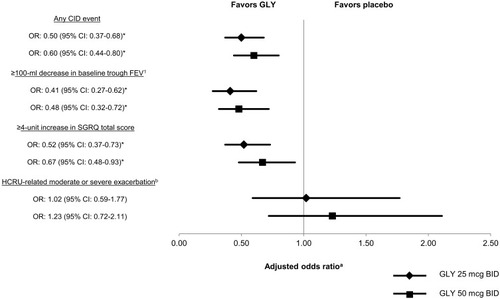
Figure 3 Subgroup analysis of the risk (adjusted odds ratio)a of CID at week 12 of treatment with GLY 25 mcg BID compared to placebo.
Abbreviations: BID, twice daily; CI, confidence interval; CID, clinically important deterioration; FEV1, forced expiratory volume in one second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; GLY, glycopyrrolate; LABA, long-acting beta-agonist; OR, odds ratio; PIFR, peak inspiratory flow rate.
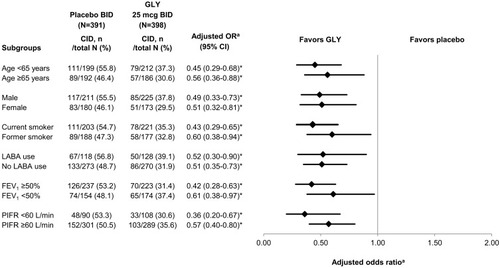
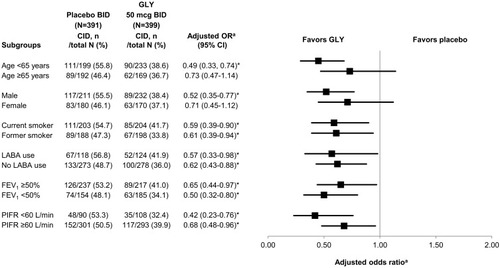
Figure 4 Subgroup analysis of the risk (adjusted odds ratio)a of CID at week 12 of treatment with GLY 50 mcg BID compared to placebo BID.
Abbreviations: BID, twice daily; CI, confidence interval; CID, clinically important deterioration; FEV1, forced expiratory volume in one second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; GLY, glycopyrrolate; LABA, long-acting beta-agonist; OR, odds ratio; PIFR, peak inspiratory flow rate.