Figures & data
Figure 1 Subject disposition.
Abbreviations: AE, adverse event; BID, twice daily; F, formoterol; MF, mometasone furoate; MF/F, mometasone furoate/formoterol fixed-dose combination formulation.
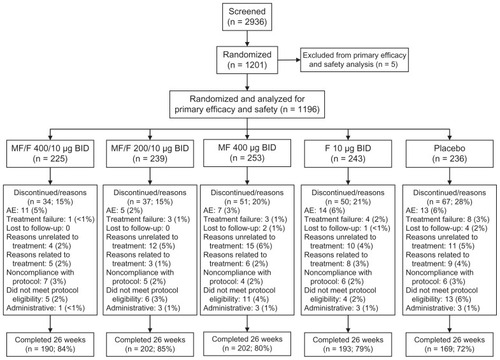
Table 1 Subject demographics and clinical characteristics
Figure 2 FEV1 AUC0–12 h at week 13 endpoint (LOCF).
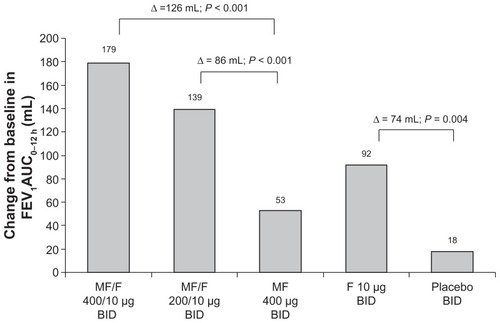
Figure 3 Serial FEV1 post-dose at day 1 (A) and week 26 (B).
Abbreviations: BID, twice daily; FEV1, forced expiratory volume in 1 second; F, formoterol; MF, mometasone furoate; MF/F, mometasone furoate/formoterol fixed-dose combination formulation.
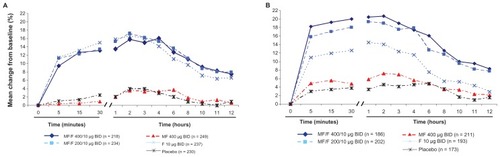
Figure 4 AM pre-dose (trough) FEV1 at week 13 endpoint.
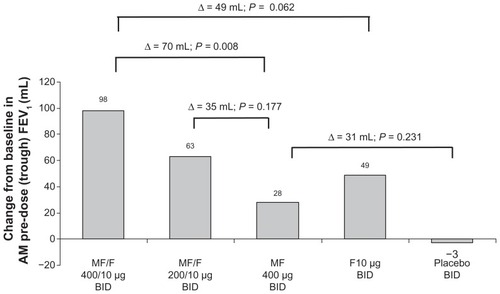
Figure 5 SGRQ total score change from baseline at week 26 endpoint.
Abbreviations: BID, twice daily; SGRQ, St George’s Respiratory Questionnaire; FEV1, forced expiratory volume in 1 second; F, formoterol; MF, mometasone furoate; MF/F, mometasone furoate/formoterol fixed-dose combination formulation.
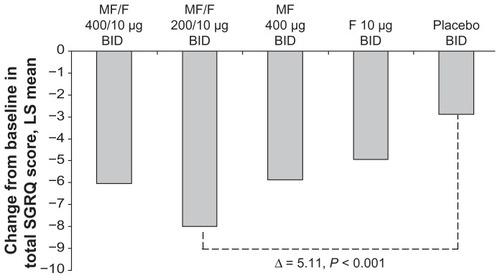
Figure 6 Time to first moderate or severe exacerbation over the 26-week treatment period.
Abbreviations: F, formoterol; MF, mometasone furoate; MF/F, mometasone furoate/formoterol fixed-dose combination formulation.
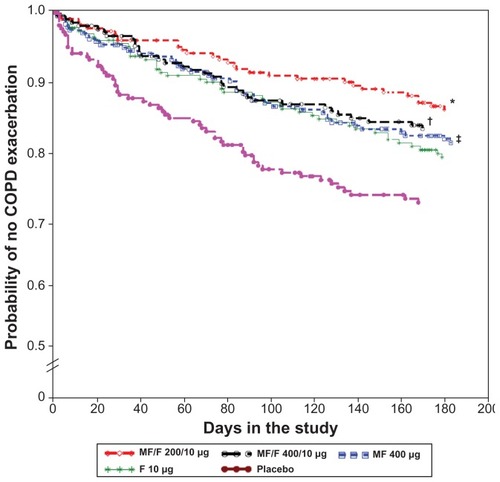
Table 2 Summary of treatment-emergent adverse events
Table 3 Treatment-emergent adverse events in ≥2% of subjects in any treatment group
Table 4 Treatment-emergent adverse events in ≥2% of subjects in any active treatment group over the safety extension