Figures & data
Table 1 Comparative Pharmacology and Pharmacokinetic Characteristics of LABAs
Figure 1 The bronchodilator effect of formoterol occurs rapidly. A single inhalation of medication was administered to 24 patients with stable COPD in a randomized, three-way, crossover study. Reprinted from Respiratory Medicine, Vol 95 (10), Benhamou et al, Rapid onset of bronchodilation in COPD: a placebo-controlled study comparing formoterol (Foradil Aerolizer) with salbutamol (Ventodisk), pages 817–821, Copyright 2001, with permission from Elsevier.Citation8
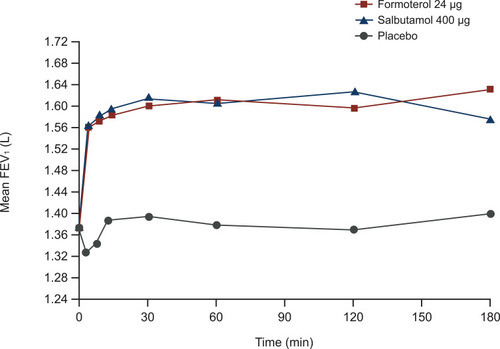
Table 2 Available Formoterol Products
Table 3 Efficacy of Formoterol in Combination with ICS
Figure 2 Time to first moderate or severe exacerbationa in the RISE study.
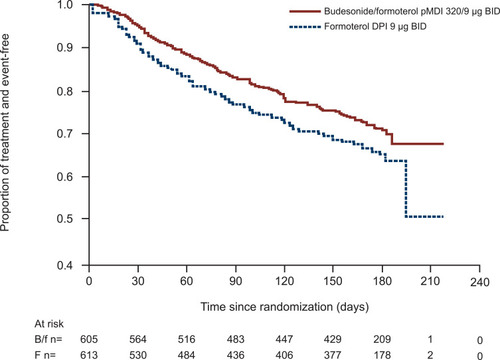
Table 4 Efficacy of Formoterol in Combination with LAMAs
Figure 3 Relative bioavailability for glycopyrronium/formoterol combination versus glycopyrronium or formoterol monotherapy. Reprinted from International Journal of Chronic Obstructive Pulmonary Disease, Vol 13, Ferguson et al, Pharmacokinetics of glycopyrronium/formoterol fumarate dihydrate delivered via metered-dose inhaler using co-suspension delivery technology in patients with moderate-to-very severe COPD, pages 945–593, Copyright 2018, with permission from Dove Medical Press.Citation26
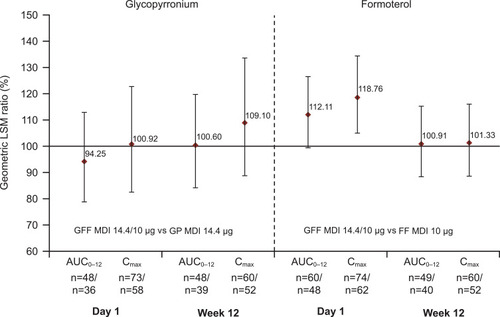
Figure 4 Effects of fixed-dose glycopyrrolate/formoterol combination on change from baseline in (A) Predose morning trough FEV1 and (B) Peak change in FEV1 within 2 hours postdose over 52 weeks versus monocomponents and open-label tiotropium. Reprinted from Respiratory Medicine, Vol 126, Hanania et al, Long-term safety and efficacy of glycopyrrolate/formoterol metered-dose inhaler using novel Co-SuspensionTM Delivery Technology in patients with chronic obstructive pulmonary disease, pages 105–115, Copyright 2017, with permission from Elsevier.Citation31
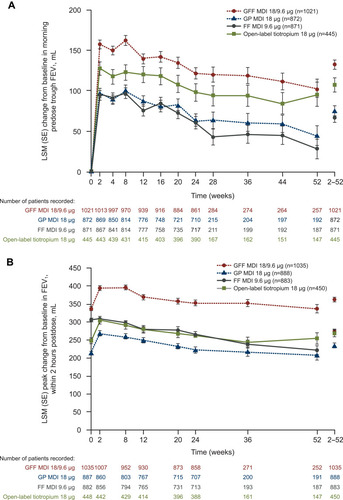
Table 5 Efficacy of Formoterol in Triple Therapy Combinations
