Figures & data
Figure 1 Inclusion criteria for EUCLID trial. This figure highlights the inclusion criteria of patients for both the EUCLID trial and this COPD-focused post hoc analysis of the EUCLID trial.
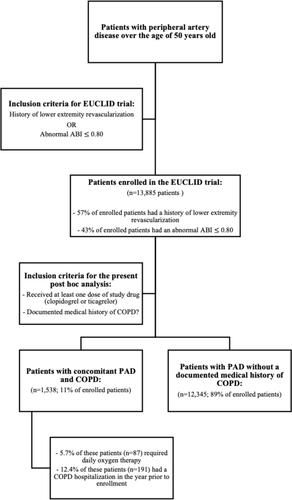
Table 1 Baseline Clinical Characteristics by COPD at Baseline
Figure 2 Kaplan–Meier plot of primary endpoint by history of COPD. The rate of occurrence of the primary endpoint (composite of cardiovascular death, myocardial infarction and ischemic stroke) is greater in patients with a history of COPD, compared to PAD patients without baseline COPD.
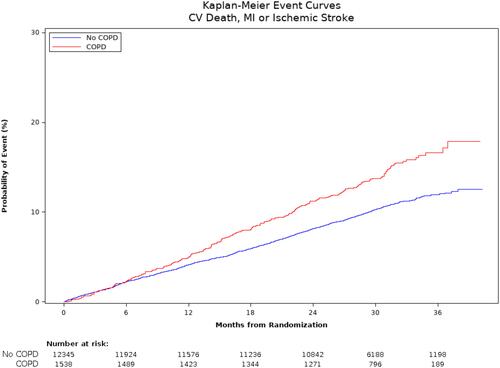
Table 2 Serious Adverse Events by History of COPD
Table 3 Adverse Events by History of COPD
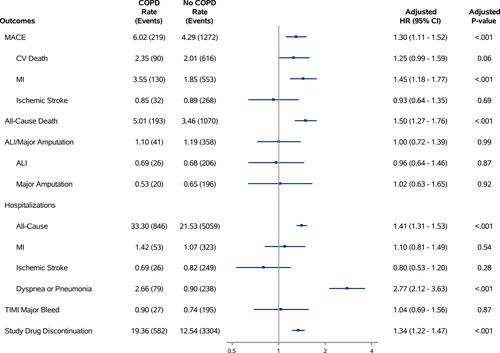
Figure 3 Association between baseline COPD and clinical outcomes. This forest plot highlights the primary efficacy outcomes (MACE and MALE), secondary endpoints of interest (all-cause mortality), safety endpoints (TIMI major bleed), and serious adverse events (all-cause hospitalizations, hospitalization for dyspnea or pneumonia, premature study drug discontinuation).