Figures & data
Figure 1 Overview of Study Design and Eligibility Criteria for the Study Cohorts. aOther COPD medications: tiotropium, other LAMA (glycopyrronium bromide, umeclidinium), LAMA/LABA (glycopyrronium/indacaterol, umeclidinium/vilanterol, and tiotropium/olodaterol), LABA (formoterol, salmeterol, indacaterol, olodaterol), and LABA/ICS (formoterol/budesonide, formoterol/beclometasone, formoterol/fluticasone, salmeterol/fluticasone propionate, vilanterol/fluticasone). bCriterion A: patients with any of the following non-cardiovascular, life-threatening conditions recorded in the database at any time before the start date: cancer, HIV (human immunodeficiency virus), respiratory failure, end-stage renal disease, organ transplant, drug or alcohol abuse, coma, or congenital anomalies. cCriterion C: prior history of hospitalisation for heart failure. dCriterion B: patients with missing information on smoking or body mass index. eChronic heart failure, causes of heart failure (ischaemic heart disease, cardiac valve disease, diseases of the myocardium, hypertension, other causes), diabetes, pulmonary embolism, asthma, hyperlipidaemia, anaemia, peripheral vascular disease, cerebrovascular diseases, stroke, transient ischaemic attack, renal disease, liver disorders. fRespiratory (SABA, oral glucocorticosteroids, mucolytics, antihistamines, ICS, SAMA, cough and cold preparations) and non-respiratory medications (antibiotics, cardiovascular medications, lipid-lowering drugs, antihypertensive medications, antiarrhythmics, nitrates, antidiabetics, and vaccines). gEarliest of outcome of interest (hospitalisation for heart failure), death, disenrollment from the practice, or end of the study period. Source: Original design diagram template can be found at www.repeatinitiative.org/projects.html.
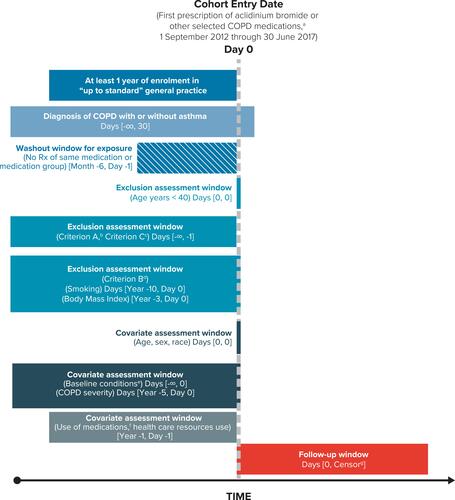
Figure 2 Cohort Attrition for New Users of Aclidinium Bromide and Other Study Medications. aPercentages are row percentages. The rest of the percentages in figure are based on the total number of all users in each column. bEligibility criteria were assessed at each prescription recorded within the study period for each user of a study medication. A patient became eligible at the date of the first prescription of the study medication that fulfilled the eligibility criteria. For those patients not included in the cohort, the eligibility criteria were assessed through their last prescription within the study period. The most restrictive criterion was a 6-month prescription-free period prior to the start date; hence, the number of eligible patients is the same in both columns. cExclusion criterion A comprises cancer or other serious, non-cardiovascular life-threatening conditions or indicators of severe comorbidity recorded in the database at any time before the start date. Exclusion criterion B comprises missing data on smoking and body mass index (2.7% of the patient-cohort users).dExclusion criterion C is prior hospitalisation for heart failure recorded in the databases any time before the start date.
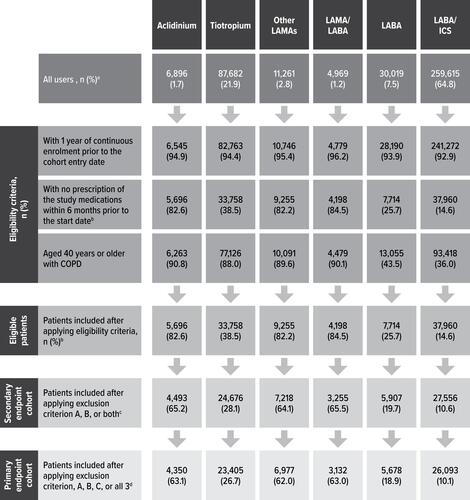
Table 1 Characteristics at the Start Date, by Study Medication, Among Patients Without Prior Hospitalisation for Heart Failure
Table 2 Crude Incidence Rate and Crude and Adjusted Incidence Rate Ratio for First-Ever Hospitalisation for Heart Failure Comparing Overall, Single, and Multiple Use of Each Study Medication with Use of Long-Acting Beta-Agonists Among Patients Without Prior Hospitalisation for Heart Failure
Figure 3 Subgroup and Sensitivity Analysis: Use of Study Medications Versus Use of LABA. aAll the models were adjusted by age, sex, COPD severity, prior outpatient diagnosis of congestive heart failure, diuretic use, ICS use, asthma, and calendar year at start date, unless one of these variables was used for stratification. bAs measured through GOLD 2016 severity categories at the start date. cWith current asthma (ie, at least one asthma diagnosis recorded within 5 years before the start date).

