Figures & data
Figure 1 Patient flow through the study.
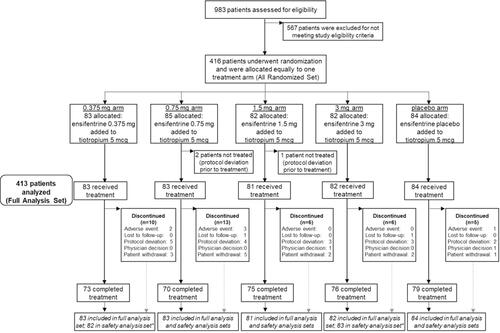
Table 1 Baseline Demographics and Disease Characteristics in the Full Analysis Set
Table 2 Baseline Patient-Reported Outcome (PRO) Scores and Rescue Medication Use for Patients with Baseline and Week 4 Values Reported in the Full Analysis Set
Table 3 Change from Baseline to Week 4 in Peak FEV1 (Over 3 Hours), Average FEV1 (0–12 Hours) and Morning Trough FEV1 in the Full Analysis Set
Figure 2 Peak FEV1 between 0 and 3 h post-dose in the full analysis set.
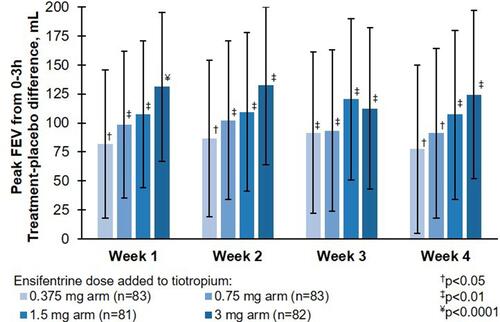
Table 4 Change from Baseline to Week 4 in SGRQ-C Total Score, E-RS™: COPD Total Score and TDI Score in the Full Analysis Set
Table 5 Proportion of Treatment Emergent Adverse Events Summary Listed by Prevalence in the Safety Analysis Set
