Figures & data
Figure 1 Study schematic (Panel A) and trial profile (Panel B).
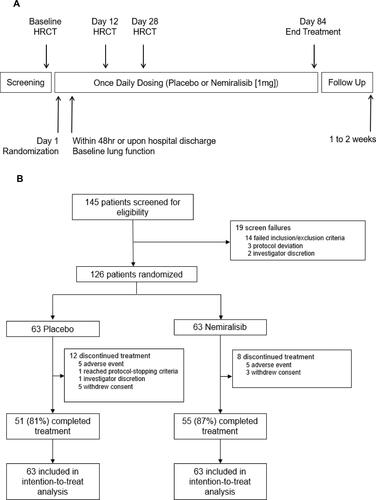
Table 3 Placebo Corrected Changes in Secondary LD-HRCT Endpoints
Table 1 Baseline Characteristics
Table 2 Summary of Adverse Events
Figure 2 Median % change from baseline in distal region siVaw at FRC after 28 days.
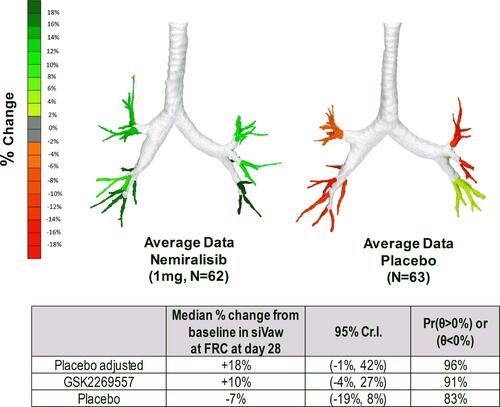
Figure 3 Placebo-corrected change in siVaw at FRC after 12 and 28 days of treatment with nemiralisib.
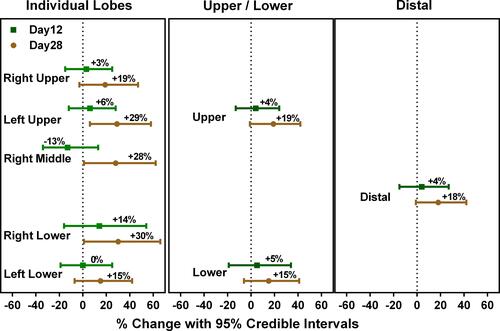
Figure 4 Change from baseline in FEV1 (Panel A) and sGaw (Panel B) in relation to the severity of the index exacerbation (FEV1 and sGaw measured at clinical visits at baseline, Day 28 and Day 84). Data presented as median ± 95% Cr I. Analyses of FEV1 data excludes data for one subject (281) due to erroneously large FEV1 recorded at baseline.
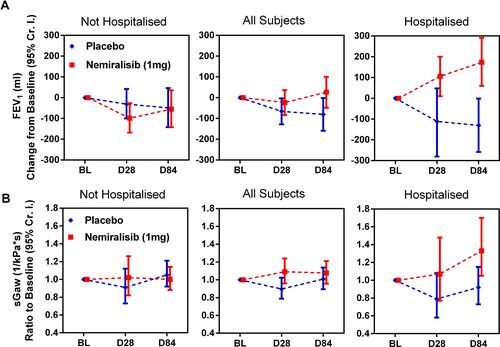
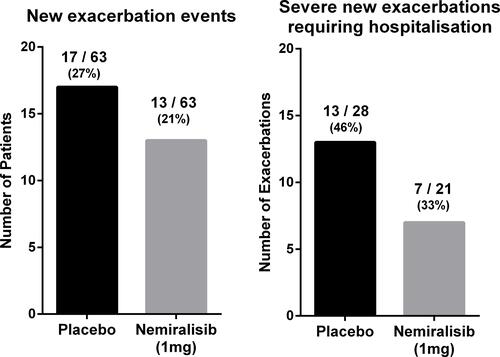
Figure 5 Number of patients (out of 63 per arm) with any new exacerbation eventsa during treatment and of the observed total number of severe exacerbationsb (28 in the placebo arm and 21 in the nemiralisib arm) the proportion requiring hospitalization. aNew exacerbation event based on observed treatment for an exacerbation; a new prescription for corticosteroids and/or antibiotics. bSevere exacerbations based on investigator-reported exacerbations resulting in hospitalization.