Figures & data
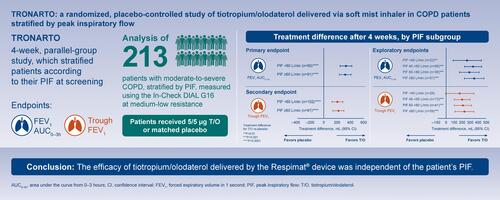
Figure 1 Patient disposition. Of the subjects in the treated set, 14 patients were excluded from the FAS (T+O, n=9; placebo, n=5) due to a lack of post-baseline efficacy measurements. The FAS (n=199) was used in the analysis of the secondary endpoint. For the primary endpoint, the FAS included n=87 patients treated with T+O and n=94 treated with placebo. Some patients excluded from the FAS also prematurely discontinued the study medication.
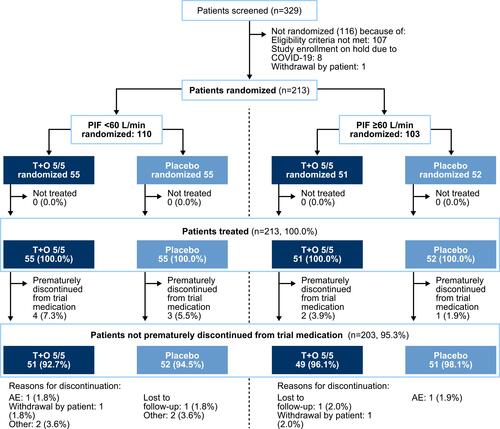
Table 1 Patient Characteristics by Treatment and by PIF
Figure 2 Treatment difference in (A) FEV1 AUC0–3h and (B) trough FEV1 after 4 weeks of treatment, by PIF subgroup (PIF ≥60 L/min vs PIF <60 L/min). FEV1 AUC0–3h analyzed using an analysis of covariance model including the fixed categorical effects of treatment and the fixed continuous effect (FEV1) of baseline. Trough FEV1 was analyzed using the restricted maximum likelihood-based approach using a mixed model with repeated measures, including the fixed, categorical effect of treatment at each visit and the fixed continuous effect (FEV1) of baseline at each visit.
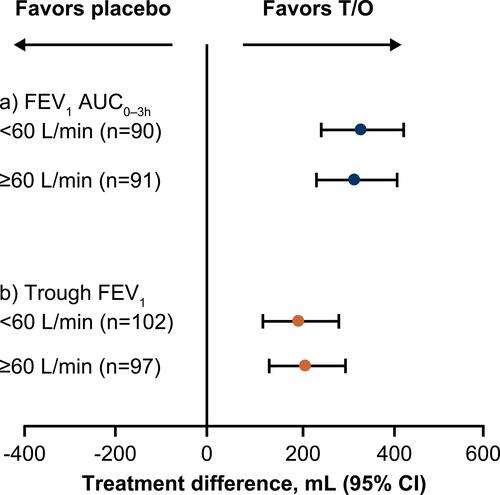
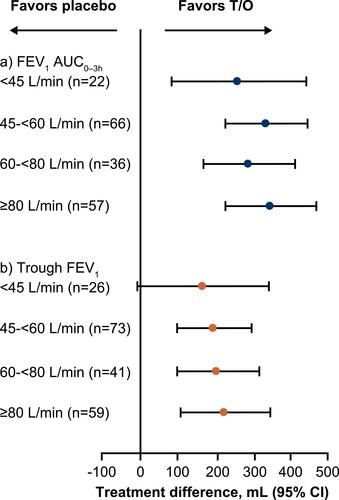
Figure 3 Treatment difference in (A) FEV1 AUC0–3h and (B) trough FEV1 after 4 weeks of treatment, by PIF subgroup (<45 L/min vs 45–<60 L/min vs 60–<80 L/min vs ≥80 L/min). FEV1 AUC0–3h analyzed using an analysis of covariance model including the fixed categorical effects of treatment and the fixed continuous effect (FEV1) of baseline. Trough FEV1 was analyzed using the restricted maximum likelihood-based approach using a mixed model with repeated measures, including the fixed, categorical effect of treatment at each visit and the fixed continuous effect (FEV1) of baseline at each visit.