Figures & data
Figure 1 Consort recruitment and retention diagram.
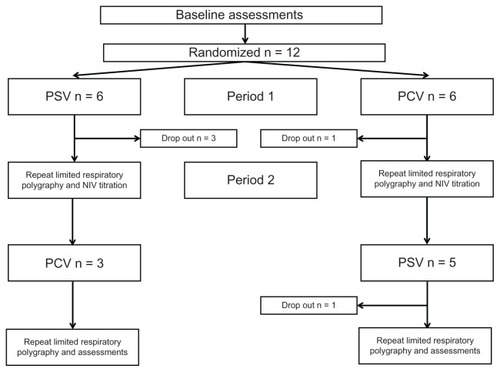
Table 1 Respiratory and ventilator parameters following 6 weeks of high-pressure and high-intensity noninvasive ventilation
Table 2 Sleep actigraphy following 6 weeks of high-pressure and high-intensity noninvasive ventilation
Table 3 Self-reported sleep comfort and sleep quality evaluated following 6 weeks of high-pressure and high-intensity noninvasive ventilation
Table 4 Severe Respiratory Insufficiency questionnaire following 6 weeks of high-pressure and high-intensity noninvasive ventilation
Figure E1 Ventilator setup protocol used at initiation of ventilation in either high-intensity or high-pressure arms.
Abbreviations: EPAP, expiratory positive airway pressure; IPAP, inspiratory positive airway pressure; NIV; noninvasive ventilation.
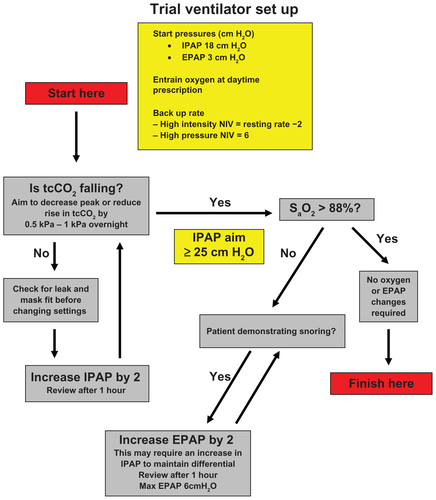
Figure E2 Individual datum points for mean nightly ventilator usage during each 6-week trial period of high-intensity or high-pressure noninvasive ventilation.
Note: Data downloaded from ventilators using Bespoke® software (B&D ElectroMedical, Warwickshire, UK).
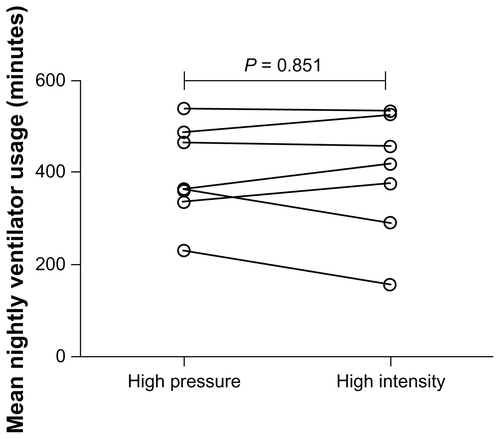
Figure E3 Individual datum points for arterial partial pressure of carbon dioxide (PaCO2) following a 6-week trial period in both the high-intensity and high-pressure arms.
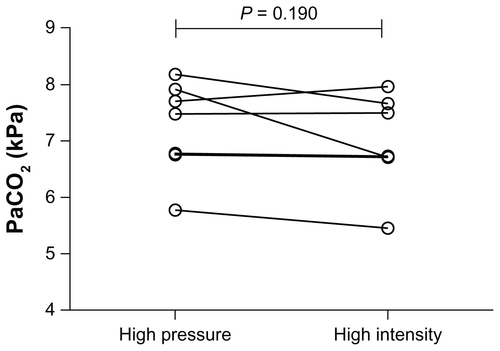
Figure E4 Individual datum points for health-related quality of life as measured by the Severe Respiratory Insufficiency questionnaire summary scale (A) and Severe Respiratory Insufficiency respiratory complaints domain (B) following 6-week trial period in the high-intensity or high-pressure arms.
Abbreviations: SRI, Severe Respiratory Insufficiecy.
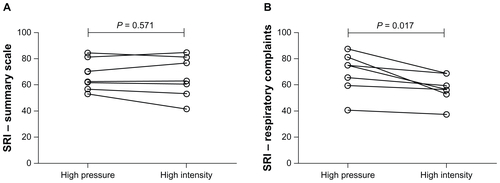
Table E1 Individual baseline characteristics for all recruited patients