Figures & data
Figure 1 Overview of the DANICO study.
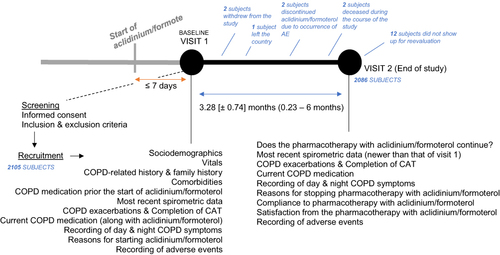
Figure 2 Summary of the different treatment pathways that were followed by the study participants, either before being prescribed with aclidinium/formoterol, at visit 1 and visit 2 of the study.
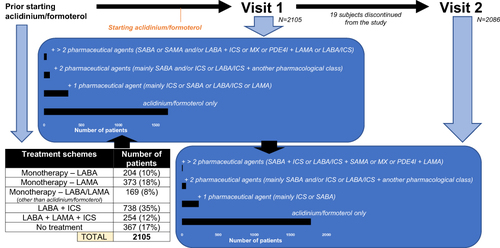
Figure 3 (A) Patient distribution according to the physician-assessed severity of early-morning at baseline and at the follow-up visit (visit 2). Numbers indicate the percentages of patients. (B) Patient distribution according to the severity of specific early-morning symptoms as perceived by the patients at baseline and at the follow-up visit (visit 2). Numbers indicate the percentages of patients.
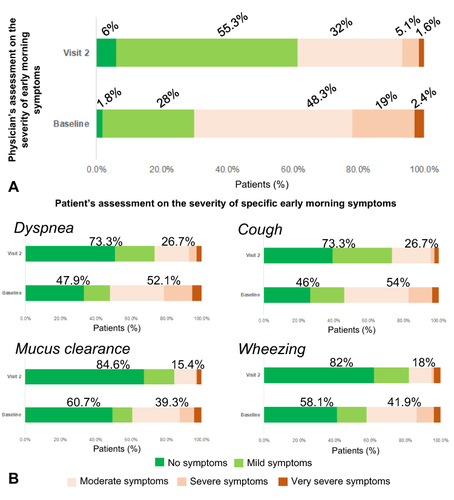
Figure 4 (A) Patient distribution according to the impact of daily COPD-related symptoms on the patients’ daily activities at baseline and at the follow-up visit (visit 2). Numbers indicate the percentages of patients. (B) Patient distribution according to the physician-assessed severity of night-time symptoms at baseline and at the follow-up visit (visit 2). Numbers indicate the percentages of patients. (C) Patient distribution according to whether the frequency of their nocturnal awakenings was reduced (improvement), increased (deterioration) or remained unchanged between the baseline and follow-up visit. Numbers indicate the percentages of patients.
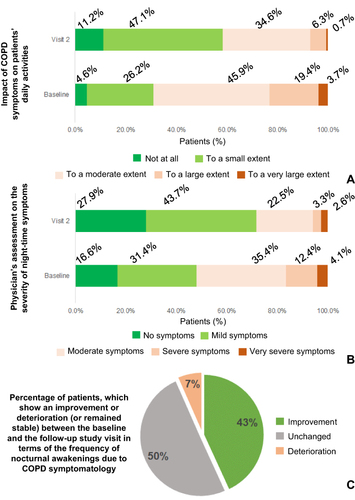
Figure 5 (Spirometric parameters - Upper panel in each ABCD group) Data shown are mean ± standard deviation. There was a statistically significant interaction between the state of bronchodilation and visit (before and after being treated with Aclidinium/formoterol) on %FEV1predicted in patients of GOLD Groups A–C (F1,226 = 5.73, p = 0.018, partial η2 = 0.025, F1,1239 = 7.09, p = 0.008, partial η2 = 0.006, and F1,199 = 7.73, p = 0.006, partial η2 = 0.037, respectively). Ιn Group A, pre-bronchodilation %FEV1predicted increased on average by 1.74%, and post-bronchodilation by 0.95%. In Group B, the corresponding percentages were 3.43% and 3.17%. In Group C, 2.9% and 2.1%. In Group D, no interaction between the study visits and the state of bronchodilation has been determined. (CAT – Lower panel in each ABCD group) Data shown are mean difference ± standard deviation. Participants on average were scoring lower to CAT in their second study visit compared to their first one, a statistically significant decrease across all GOLD ABCD groups (−1.69 with 95% CI [−2.16 to −1.21], t234 = −6.94, p < 0.001, d = 0.45 for Group A, −5.2 with 95% CI [−5.47 to −4.93], t1271 = −37.84, p < 0.001, d = 1.06 for Group B, −5.93 with 95% CI [−6.91 to −4.96], t208 = −11.99, p < 0.001, d = 0.83 for Group C and −7.15 with 95% CI [−7.78 to −6.52], t369 = −22.25, p < 0.001, d = 1.16 for Group D).
![Figure 5 (Spirometric parameters - Upper panel in each ABCD group) Data shown are mean ± standard deviation. There was a statistically significant interaction between the state of bronchodilation and visit (before and after being treated with Aclidinium/formoterol) on %FEV1predicted in patients of GOLD Groups A–C (F1,226 = 5.73, p = 0.018, partial η2 = 0.025, F1,1239 = 7.09, p = 0.008, partial η2 = 0.006, and F1,199 = 7.73, p = 0.006, partial η2 = 0.037, respectively). Ιn Group A, pre-bronchodilation %FEV1predicted increased on average by 1.74%, and post-bronchodilation by 0.95%. In Group B, the corresponding percentages were 3.43% and 3.17%. In Group C, 2.9% and 2.1%. In Group D, no interaction between the study visits and the state of bronchodilation has been determined. (CAT – Lower panel in each ABCD group) Data shown are mean difference ± standard deviation. Participants on average were scoring lower to CAT in their second study visit compared to their first one, a statistically significant decrease across all GOLD ABCD groups (−1.69 with 95% CI [−2.16 to −1.21], t234 = −6.94, p < 0.001, d = 0.45 for Group A, −5.2 with 95% CI [−5.47 to −4.93], t1271 = −37.84, p < 0.001, d = 1.06 for Group B, −5.93 with 95% CI [−6.91 to −4.96], t208 = −11.99, p < 0.001, d = 0.83 for Group C and −7.15 with 95% CI [−7.78 to −6.52], t369 = −22.25, p < 0.001, d = 1.16 for Group D).](/cms/asset/334d0c11-ee30-4677-be65-424b1c427384/dcop_a_12152869_f0005_c.jpg)
Figure 6 (A) Reasons for prescribing Aclidinium/formoterol by the treating physicians. (B) Satisfaction of patients using Aclidinium/formoterol per GOLD ABCD group. (C) Compliance to the treatment scheme with Aclidinium/formoterol, and reasons for missing doses.
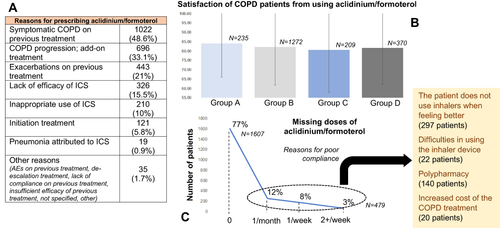
Table 1 The 6 Cases of Adverse Events Having Occurred During the Observational Study
