Figures & data
Table 1 List of Genetic Primers
Table 2 Interference and Negative Control Sequence
Table 3 Lung Function
Figure 1 ACSL4 is highly expressed in the lung tissue of COPD mice. (A) Relative expression of ACSL4 mRNA in mouse lung tissue by real-time PCR. (B) The expression of ACSL4 protein and ferroptosis-related proteins GPX4 and FTH in COPD mouse lung tissue was detected by Western blotting. (C–E) HE staining of sections of mouse lung tissue. The DI and MLI values of each group were calculated. Data are presented as the mean ± SD of three replicates and analyzed using one-way ANOVA followed by post-hoc multiple comparisons. (nsp > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001).
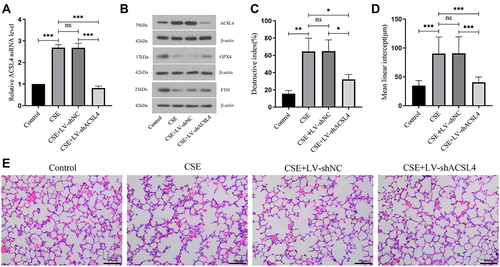
Figure 2 ACSL4 induces ferroptosis in lung tissue of COPD mice. (A–C) Lung tissue from each group of mice was analysed for markers related to ferroptosis. (D) Iron deposition in lung tissue from mice in each group was observed by Prussian blue staining. (E) Transmission electron microscopy revealed the mitochondrial morphology in lung tissue from mice in each group; red arrows indicate mitochondria. Data are presented as the mean ± SD of three replicates and analyzed using one-way ANOVA followed by post-hoc multiple comparisons. (nsp > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001).
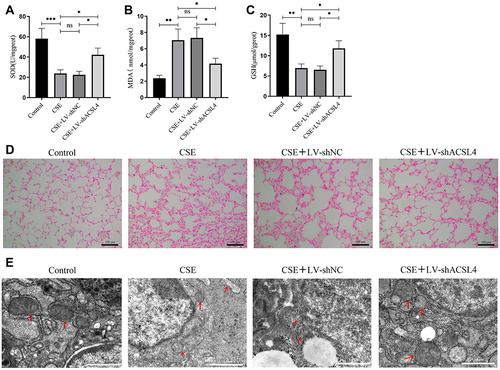
Figure 3 ACSL4 induces ferroptosis in COPD lung epithelial cells. (A) CCK-8 determination of cell viability in each group. (B) Detection of iron ion content in cells of each group. (C) Lipid ROS content of cells in each group. (D) Relative expression levels of ACSL4 mRNA in each cell group. (E) ACSL4 protein expression in cells of each group. (F and G) Three siRNAs against human ACSL4 and control NC were constructed and transferred into BEAS-2B cells using transfection reagents. Real-time PCR and Western blotting were used to detect the ACSL4 mRNA and protein expression in the cells, respectively, and the siRNAs with the best interference efficiency were screened. Data are presented as the mean ± SD of three replicates and analyzed using one-way ANOVA followed by post-hoc multiple comparisons. (nsp > 0.05, **p < 0.01, ***p < 0.001).
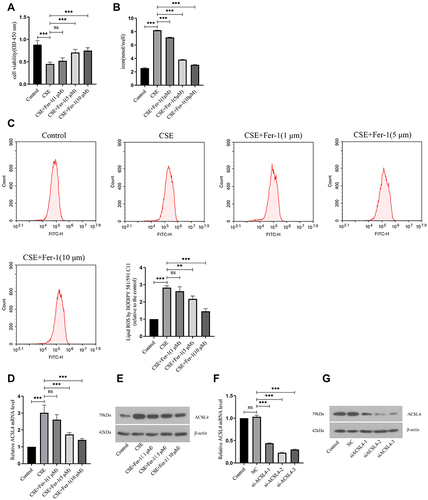
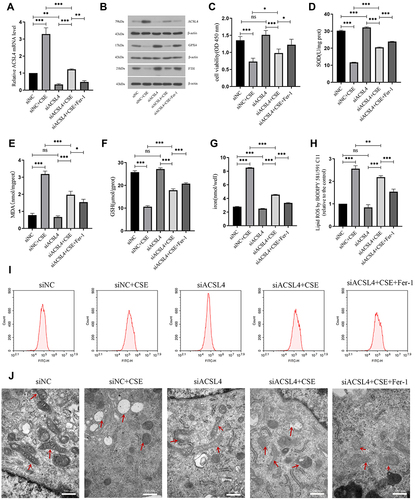
Figure 4 Interference with ACSL4 can alleviate ferroptosis in lung epithelial cells. (A) Relative expression level of ACSL4 mRNA in each group of cells after siRNA interference. (B) ACSL4, GPX4 and FTH protein expression in each group after siRNA interference. (C) CCK-8 detection of cell viability in each group. (D–F) Content detection of the ferroptosis related markers SOD, MDA and GSH. (G) Detection of iron ion content in cells of each group. (H and I) Detection of lipid ROS content by the BODIPY 581/591 C11 probe method. (J) Observation of mitochondrial morphology in cells of each group by transmission electron microscopy. Red arrows indicate mitochondria. Data are presented as the mean ± SD of three replicates and analyzed using one-way ANOVA followed by post-hoc multiple comparisons. (nsp > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001).