Figures & data
Figure 1 Study design.
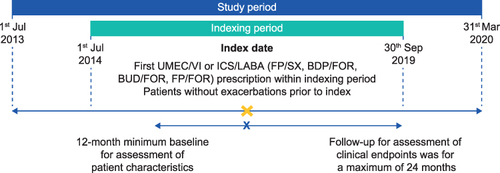
Table 1 Patient Demographics and Clinical Characteristics
Figure 2 (A) Proportion of patients and (B) odds of adherence (PDC≥80%) at 12 months post-index.
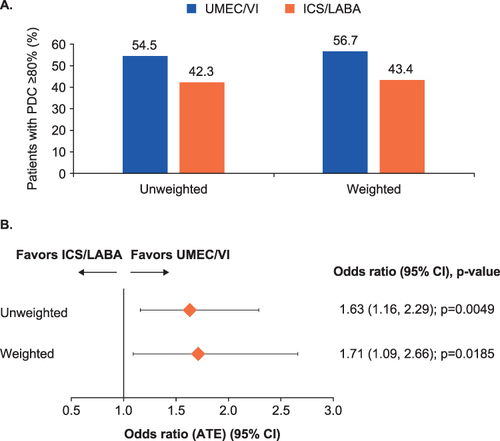
Figure 3 Odds of adherence (PDC≥80%) at 6, 18, and 24 months post-index.
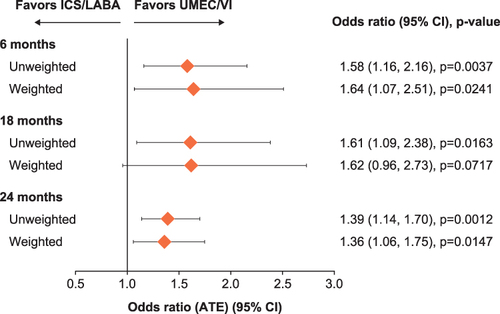
Figure 4 (A) Time-to-first triple therapy and (B) time-to-first moderate-to-severe COPD exacerbation.
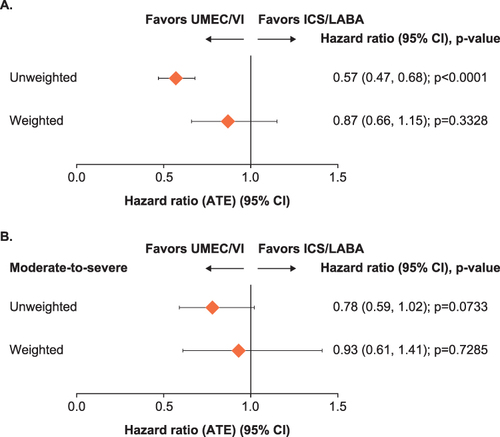
Figure 5 Rate ratio of (A) COPD-related and (B) all-cause HCRU use at 12 months post-index.
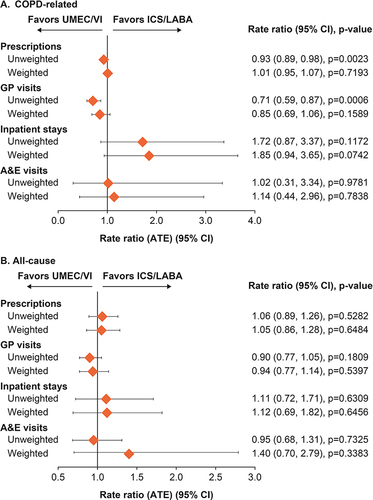
Figure 6 (A) COPD-related and (B) all-cause direct healthcare costs at 12 months post-index.
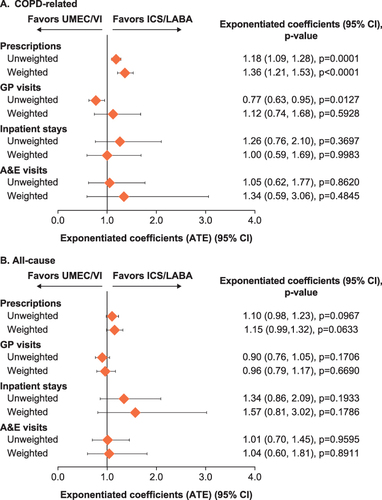
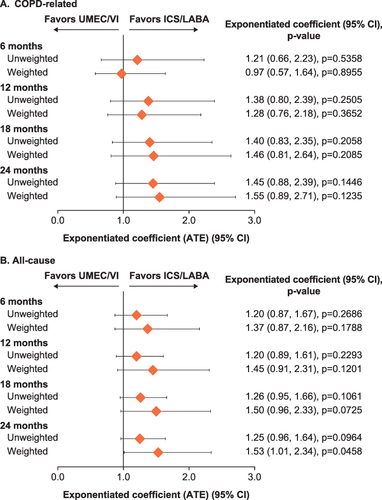
Figure 7 (A) COPD-related and (B) all-cause and total costs at 6, 12, 18, and 24 months post-index.