Figures & data
Figure 1 Ensifentrine mechanism of action. Ensifentrine is a novel inhaled, single molecule that is a potent and selective inhibitor of PDE3 and PDE4 in late-stage clinical development for treatment of patients with COPD. PDE3 and PDE4 are expressed on airway smooth muscle, inflammatory cells and bronchial epithelial cells. The dual inhibition of PDE3 and PDE4 by ensifentrine produces additive or synergistic effects compared with inhibition of either PDE3 or PDE4 alone, which results in enhanced effects on bronchodilation, airway inflammation, and mucociliary clearance.
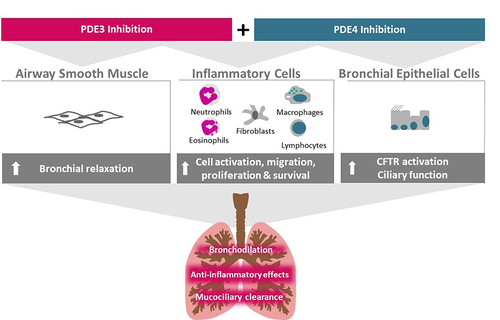
Table 1 Summary of Clinical Study Designs of Nebulized Ensifentrine in Healthy Volunteers and in Subjects with COPD
Table 2 Summary of Phase 2 Clinical Study Efficacy Data of Nebulized Ensifentrine by Dose in Subjects with COPD
Table 3 The Most-Commonly Reported AEs (by More Than One Subject) with Nebulized Ensifentrine (3 mg) Twice Daily in Subjects with COPD from Two 4-Week Phase 2 Dose-Ranging Studies RPL554-CO-203 and RPL554-CO-205a,Citation32
