Figures & data
Table 1 Therapies That Inhibit Recruitment and Activation of the Cellular Components of Inflammation in COPD
Figure 1 Effect of PDE inhibition in COPD. Combined inhibition of PDE3 and PDE4 provides additive and synergistic anti-inflammatory and bronchodilator effects when compared to PDE3 or PDE4 inhibition alone. It also improves mucociliary clearance. The primary PDE implicated in the activity of the given cell is shown in red.
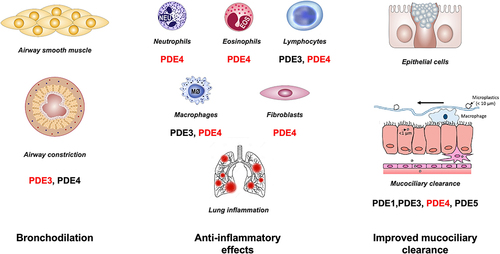
Figure 2 The role of p38 MAPK in the pathobiology of COPD. At the level of alveolar macrophages and other inflammatory cells, airborne pollutants, cigarette smoke, and microbial pathogens activate p38 MAPK. The p38 signaling pathway leads to increased cytokine and chemokine production, particularly interleukin (IL)-1β, IL-8, and tumor necrosis factor-α (TNF-α), which are associated with the neutrophilic endotype of COPD. Therefore, inhibiting p38 MAPK may be an effective treatment for patients with COPD.
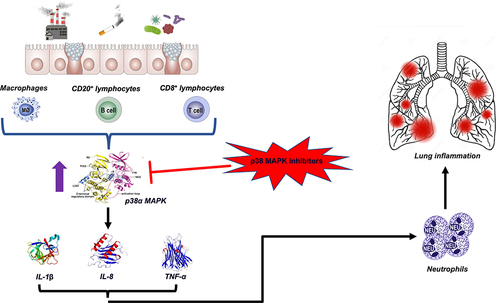
Figure 3 Monoclonal antibodies targeting T2 cytokines in COPD.
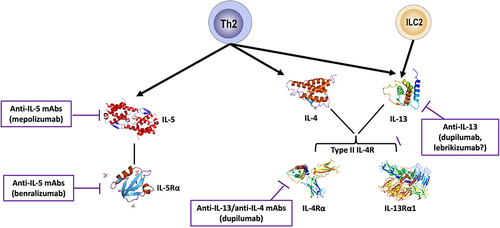
Figure 4 Targeting alarmins in COPD.
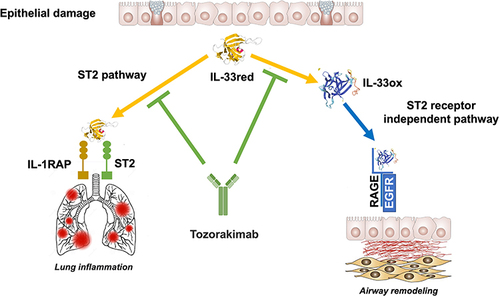
Table 2 Therapies That Antagonize the Products of the Cellular Components of Inflammation in COPD
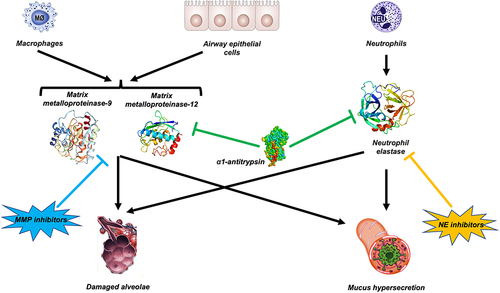
Figure 5 Proteolytic interplay between α1-antitrypsin (AAT), matrix metalloproteinases (MMPs) and neutrophil elastase (NE). Several proteases, including neutrophil elastase (NE) and MMP-9 and MMP-12, are involved in COPD. Therefore, inhibiting a single enzyme with a NE inhibitor or a MMP inhibitor may not have a significant therapeutic impact. AAT inhibits NE and reduces macrophage MMP-12 synthesis.