Figures & data
Table 1 Participant Demographics and Characteristics (N = 20)
Figure 2 Geometric Mean Plasma Concentration (pg/mL) of (A) Aclidinium, (B) LAS34823 and (C) LAS34850 Versus Time: Day 1 and Day 9.
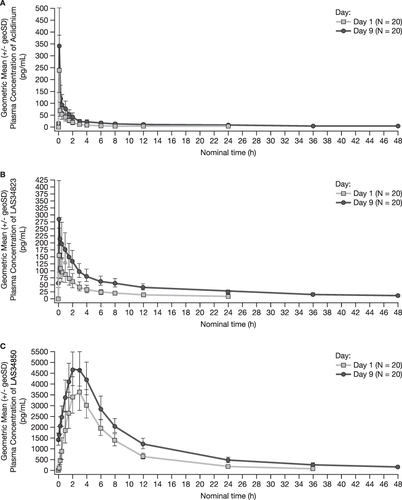
Table 2 Pharmacokinetic Parameters of Aclidinium Bromide and Its Metabolites LAS34823 and LAS34850, Following a Single Dose (Day 1) and Following Multiple Dosing (Day 9 After 5 Days of Repeated BID Dosing) of Aclidinium Bromide 400 μg (N = 20)