Figures & data
Table 1 Patient baseline demography and disease characteristics (FAS population; N = 4435)
Figure 1 Disposition of study patients.
Figure 2 Summary change from baseline in SGRQ total scores, activity domain, impact domain, and symptom domain (FAS population).
Abbreviations: FAS, full analysis set; SGRQ, St George’s Respiratory Questionnaire.
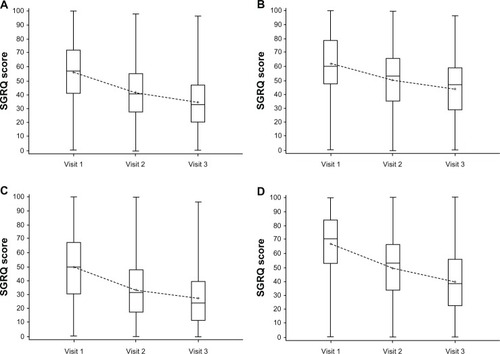
Figure 3 Summary change from baseline in SGRQ total scores among COPD patients.
Abbreviations: COPD, chronic obstructive pulmonary disease; CR, Croatia; PL, Poland; RO, Romania; RU, Russian Federation; SD, standard deviation; SGRQ, St George’s Respiratory Questionnaire; SI, Slovenia; SK, Slovakia.
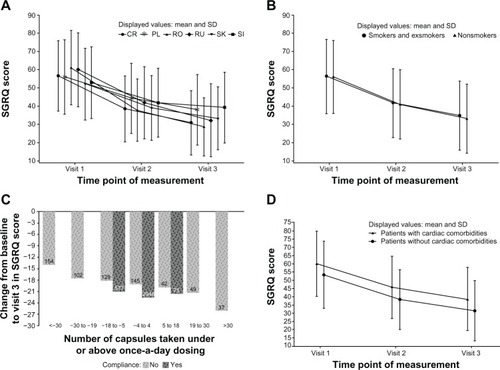
Table 2 Summary of patients SGRQ status and the association between characteristic of patients and relative improvement in SGRQ score within 6 months – FAS
Table 3 Summary of reported AEs (TS population; N = 4852)