Figures & data
Table 1 Study characteristics of included trials that provide data on the total number of exacerbations and/or the mean annual rate of exacerbations
Figure 2 Diagram displaying the network of eight arms involved in the Bayesian analyses.
Abbreviations: BDP, beclomethasone dipropionate; BUD, budesonide; FF, fluticasone furoate; FM, formoterol; FP, fluticasone propionate; ICS, inhaled corticosteroids; LABA, long-acting beta agonists; MF, mometasone furoate; PLB, placebo; SAL, salmeterol; VI, vilanterol.
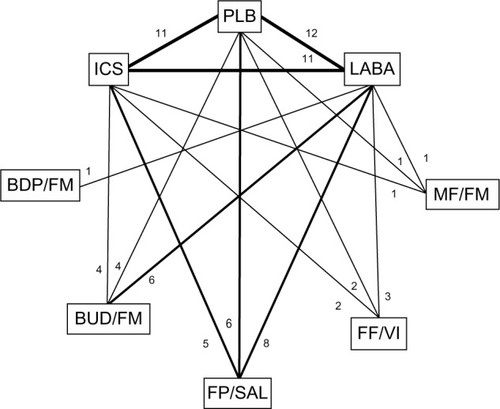
Figure 3 Pooled effect estimate on moderate-to-severe exacerbations for all combined inhalers versus placebo.
Abbreviations: BDP, beclomethasone dipropionate; BUD, budesonide; FF, fluticasone furoate; FM, formoterol; FP, fluticasone propionate; MF, mometasone furoate; SAL, salmeterol; VI, vilanterol.
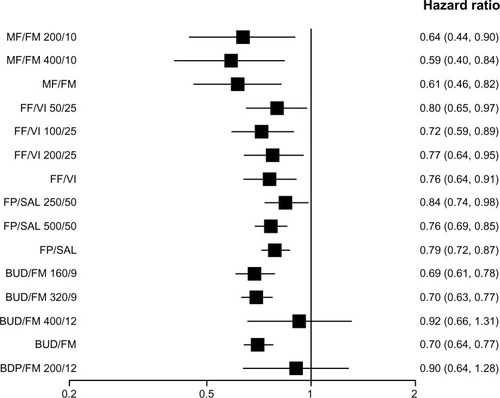
Figure 4 Pooled effect estimate on moderate-to-severe exacerbations for all combined inhalers versus long acting beta-agonist.
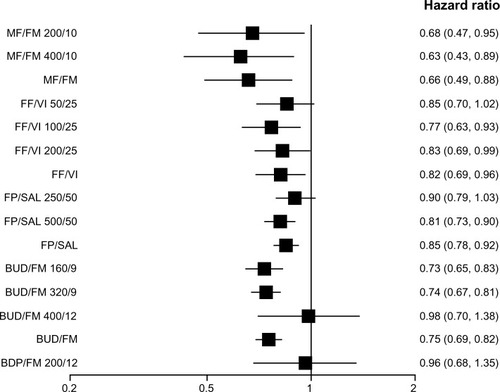
Figure 5 Pooled effect estimate on severe exacerbations for all combined inhalers versus placebo.
Abbreviations: BDP, beclomethasone dipropionate; BUD, budesonide; FF, fluticasone furoate; FM, formoterol; FP, fluticasone propionate; MF, mometasone furoate; SAL, salmeterol; VI, vilanterol.
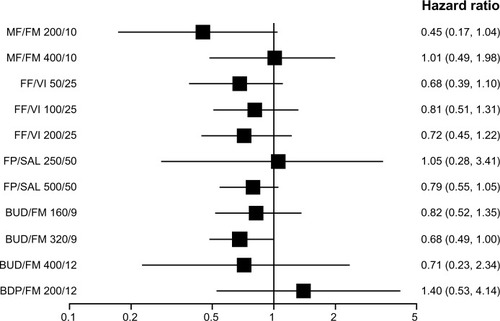
Figure 6 Pooled effect estimate on severe exacerbations for all combined inhalers versus long acting beta-agonist.
Table S1 Definitions of COPD exacerbations in the included trials