Figures & data
Table 1 Patient characteristics
Figure 1 Initial questionnaire on usage, effectiveness, inhalation technique, and overall satisfaction 8 weeks after switching from the HandiHaler to the Respimat inhaler.
Notes: Question 1, Do you think that you can use Spiriva well? Question 2: What do you think about the effect of Spiriva on shortness of breath or dyspnea? Question 3: Please tell us about any difficulties you experienced when using each inhaler as follows: Q3–1, on inhalation technique; Q3–2, on breath-holding technique; Q3–3, on overall handling or usability. Question 5: Please tell us your overall satisfaction with Spiriva for each inhaler. The inhalation technique required for Respimat was significantly more difficult than that required for the HandiHaler (P=0.049, question 3–1), but there was no significant difference in the usage (question 1), perceived effect of the medication (question 2), breath-holding technique (question 3–2), or overall handling (question 3–3). Evaluations of the Respimat were classified as “good” or “bad” (question 5), but no significant difference was detected between the devices.
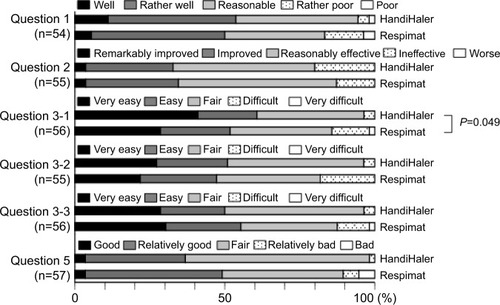
Figure 2 Questionnaire on adverse events 8 weeks after switching from the HandiHaler inhaler to the Respimat inhaler.
Notes: Respimat had a significantly milder aftertaste than the HandiHaler (P=0.004), but there were no other significant differences in the incidence of adverse events (question 4: Please indicate whether you experienced any of the following symptoms after using each device).
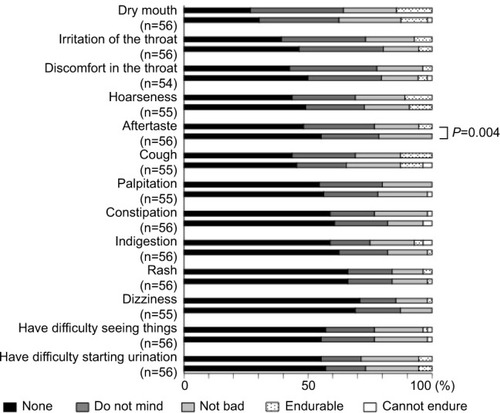
Figure 3 Comparison of patient preference for the HandiHaler and Respimat.
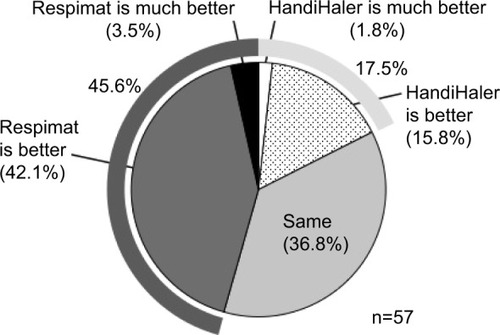
Table 2 Pulmonary function before and after switching from the HandiHaler to the Respimat
Figure 4 The second questionnaire administered 2–3 years after switching from the HandiHaler to the Respimat, and comparison of responses to those in the first survey.
Notes: Question 1: Do you think that you can use Spiriva well? Question 3: Please tell us about any difficulties you experienced when using each inhaler, as follows: Q3–2, on breath-holding technique; Q3–3, on overall handling or usability. Question 5: Please give us your overall satisfaction with Spiriva for each device. The usage (question 1), perceived breath-hold technique (question 3–2), overall handling (question 3–3), and overall satisfaction (question 5) with Respimat were significantly improved compared to the results of the first survey (P=0.0008, P=0.0017, P=0.031, and P=0.0086, respectively). The overall satisfaction with the HandiHaler was significantly worse in the second survey (P=0.0436, question 5).
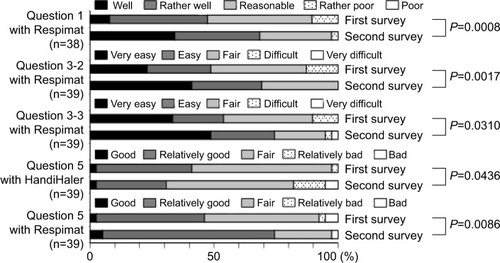
Figure 5 Changes in preference for Respimat between the first and second surveys.
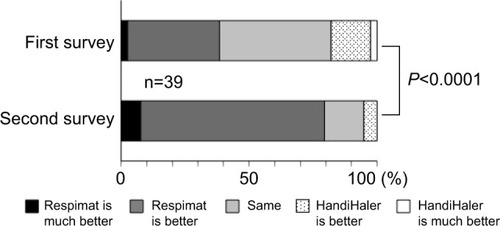
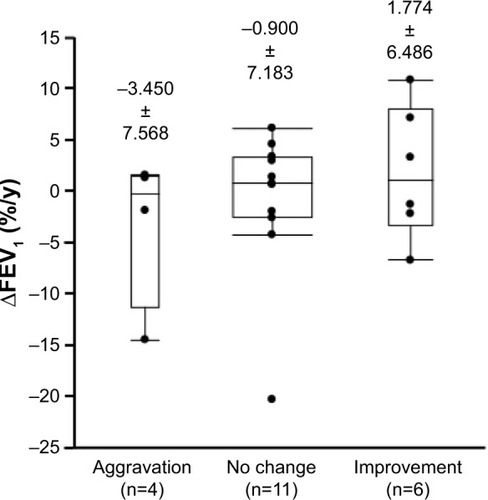
Figure 6 Annual decline in FEV1 in patients classified according to the change in overall satisfaction with each device.
Abbreviation: ∆FEV1, decline in forced expiratory volume in 1 second.