Figures & data
Table 1 Clinical trials included in the pooled analysis
Table 2 Patient baseline characteristics
Table 3 IRs (per 100 patient-years) and RRs for all AEs, by subgroup
Table 4 IRs (per 100 patient-years) and RRs for cardiac, vascular, and respiratory AEs
Table 5 IRs (per 100 patient-years) and RRs for cardiac, vascular, and respiratory SAEs
Table 6 IRs (per 100 patient-years) and RRs for MACE, by inhaler type
Table 7 IRs (per 100 patient-years) and RRs for potential anticholinergic AEs
Table 8 IRs (per 100 patient-years) and RRs for potential anticholinergic SAEs
Figure 1 Number of events, time at risk, and RRs for all-cause FAEs.
Abbreviations: BI, Boehringer Ingelheim; CI, confidence interval; FAE, fatal adverse event; RR, rate ratio.
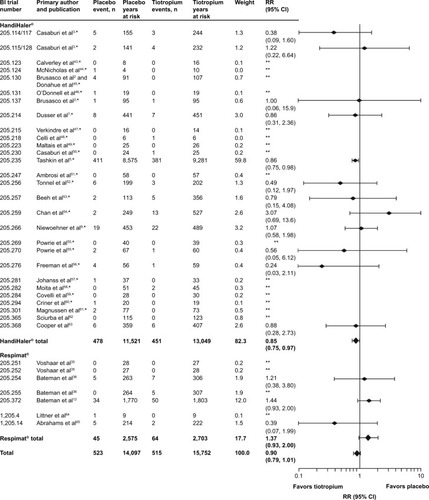
Table 9 IRs (per 100 patient-years) and RRs for FAEs according to SOC and PTTable Footnotea
Table S1 PV endpoint definition
