Figures & data
Table 1 Characteristics, laboratory findings, and pulmonary function tests of study groups
Figure 1 Serum soluble urokinase-type plasminogen activator receptor (suPAR) in healthy controls and in patients with acute exacerbation of chronic obstructive pulmonary disease (AE-COPD) on the first and seventh days.
Notes: Data are expressed as boxplots, in which the horizontal lines illustrate the 25th, 50th, and 75th percentiles of the values of suPAR. The vertical lines represent the 5th and 95th percentiles.
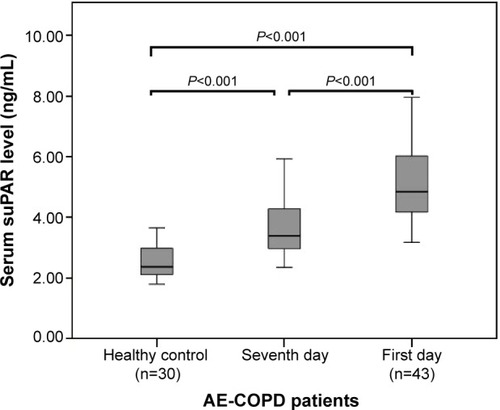
Figure 2 Serum soluble urokinase-type plasminogen activator receptor (suPAR) during an acute exacerbation and on the seventh day of treatment.
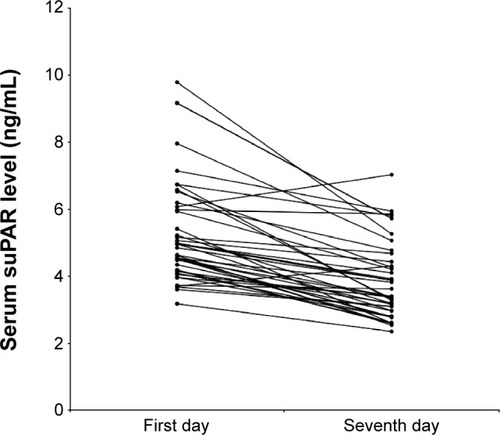
Figure 3 Serum suPAR and FEV1 % of predicted in patients with acute exacerbation of chronic obstructive pulmonary disease.
Abbreviations: FEV1, forced expiratory volume in 1 second; ln, natural log-transformed; suPAR, soluble urokinase-type plasminogen activator receptor.
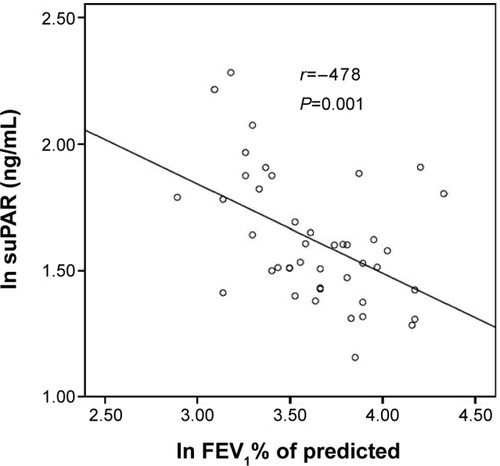
Figure 4 C-reactive protein (CRP) (A) and fibrinogen (B) in healthy subjects and patients with acute exacerbation of chronic obstructive pulmonary disease (AE-COPD) on the first and the seventh day.
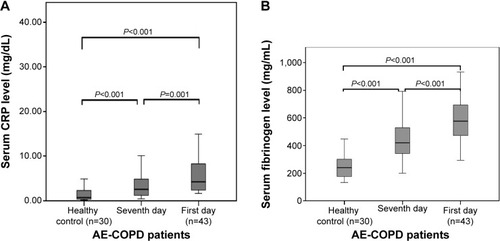
Figure 5 Serum C-reactive protein (CRP) (A) and fibrinogen (B) during an acute exacerbation and on the seventh day of the treatment.
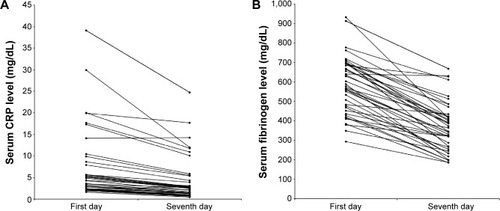
Table 2 Pearson correlation coefficients of suPAR with inflammatory markers in patients with AE-COPD on the first and seventh day
Figure 6 ROC curve for suPAR, CRP, and fibrinogen in discrimination of patients with acute exacerbation of chronic obstructive pulmonary disease on the first and seventh day.
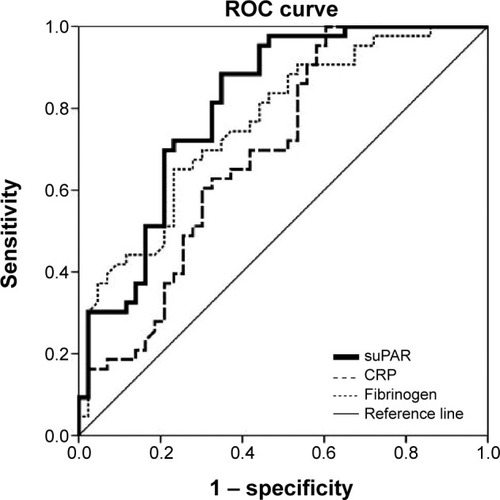
Table 3 Univariate and multivariate logistic regression analysis of inflammatory markers in patients with acute exacerbation of chronic obstructive pulmonary disease as the dependent variable
