Figures & data
Figure 1 CONSORT (consolidated standards of reporting trials) flow diagram.
Abbreviations: AE, adverse event; COPD, chronic obstructive pulmonary disease; ITT, intent-to-treat; PP, per-protocol; SFC, salmeterol/fluticasone propionate.
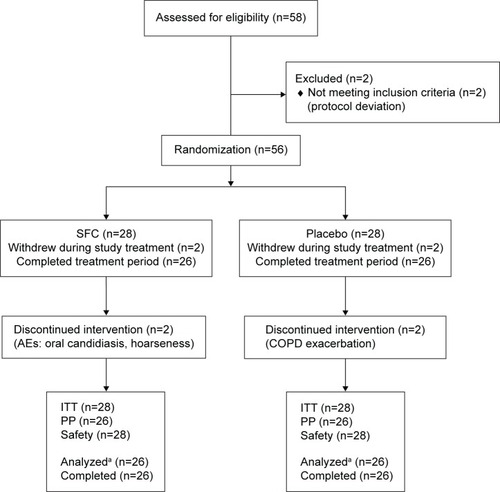
Table 1 Summary of demographic characteristics (per-protocol population)
Table 2 Statistical analysis of change from baseline in differential neutrophil count in induced sputum at week 12
Table 3 Median difference at week 12, between SFC 250 and placebo, in the change from baseline in levels of biomarkers in sputum and serum (per-protocol population)
Table 4 Median of % change from baseline neutrophils and IL-8, in sputum
Table 5 Statistical analysis of COPD pulmonary function tests at week 12 (per-protocol population)
