Figures & data
Table 1 Participating patients (n, %) by country and treatment
Table 2 Patient demographics and baseline characteristics
Figure 1 Changes from baseline in CCQ overall score.
Abbreviations: CCQ, clinical COPD questionnaire; LABA, long-acting β2-agonist.
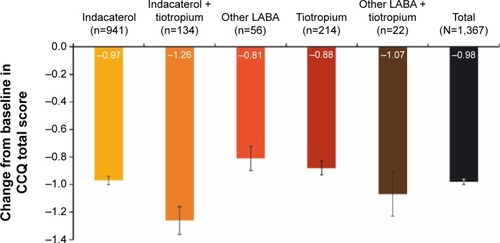
Figure 2 Patient satisfaction with current treatment at end of study.
Notes: aPatients who changed treatment during the observational period; treatment satisfaction was assessed until the time of treatment change in these patients.
Abbreviation: LABA, long-acting β2-agonist.
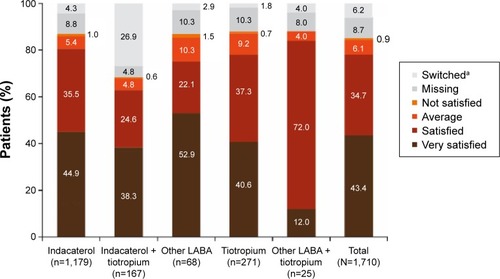
Figure 3 Physician satisfaction with current treatment at end of study.
Abbreviation: LABA, long-acting β2-agonist.
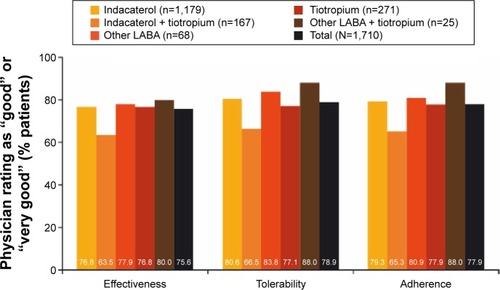
Figure 4 Patient assessment of ease of use of indacaterol inhaler at end of study.
Notes: aPatients who changed treatment during the observational period; inhaler ease of use was assessed until the time of treatment change in these patients.
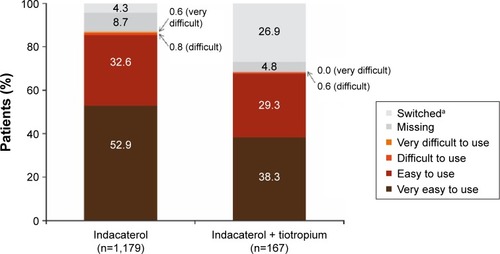
Figure 5 Physician assessment of indacaterol inhaler ease of use at end of study.
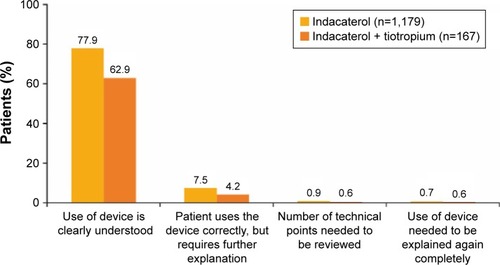
Table 3 Patients (n, %) with AEs and SAEs
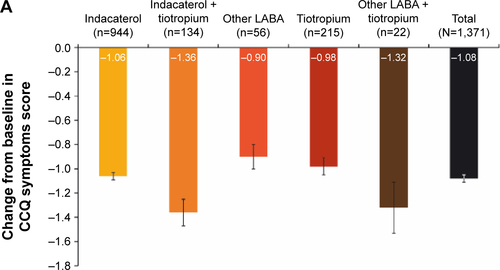
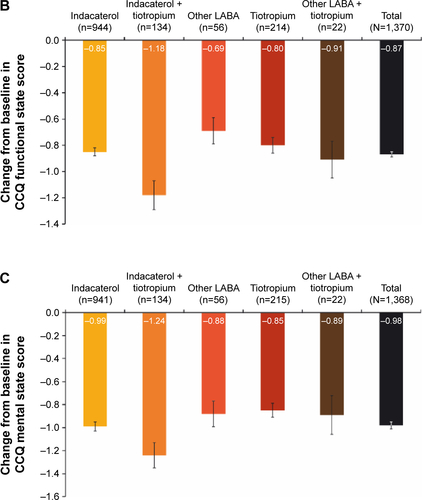
Figure S2 Change from baseline in CCQ (A) symptoms score, (B) functional state score and (C) mental state score.
Notes: Data are mean ± standard error. Last observation was carried forward. All P<0.0001 for change in CCQ from baseline to end of study. Per-protocol population.
Abbreviations: CCQ, clinical COPD questionnaire; LABA, long-acting β2-agonist.