Figures & data
Table 1 Key study characteristics for all studies included in the NMA (only arms of interest)
Figure 1 Summary of study-flow (A) registries (B) study selection.
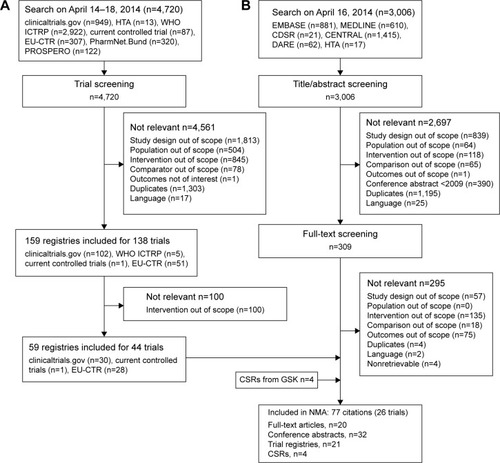
Table 2 Key patient characteristics at baseline for all studies included in the NMA (only arms of interest)
Figure 2 Overall network of studies in the NMA analysis of UMEC/VI versus LABA/LAMA combination therapies evaluated at 24 weeks for (A) trough FEV1, (B) SGRQ total score, (C) TDI focal score, and (D) rescue medication use.
Abbreviations: CI, confidence interval; FEV1, forced expiratory volume in 1 second; FOR, formoterol; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; NMA, network meta-analysis; PBO, placebo; QVA149, indacaterol/glycopyrronium; SAL, salmeterol; SD, standard deviation; SE, standard error; SGRQ, St George’s Respiratory Questionnaire; TDI, transitional dyspnoea index; TIO, tiotropium; UMEC, umeclidinium; VI, vilanterol.
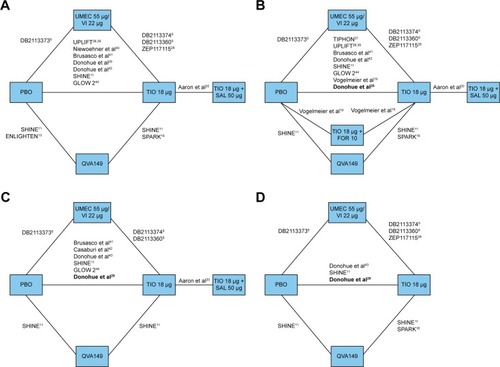
Table 3 Individual study results at 12 weeks and 24 weeks for trough FEV1, SGRQ total scores, TDI focal scores, and rescue medication use (puffs/day)
Table 4 Results of the NMA
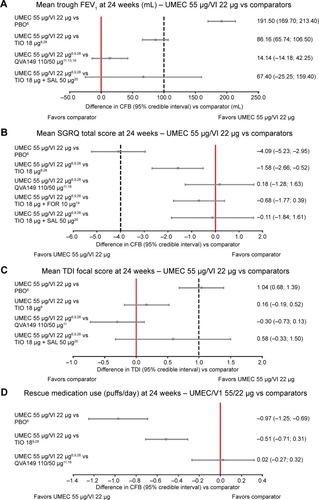
Figure 3 Forest plot for (A) mean trough FEV1, (B) mean SGRQ total scores, (C) mean TDI focal scores, and (D) mean rescue medication use of UMEC 55 μg/VI 22 μg versus comparators at 24 weeks.
Abbreviations: CFB, change from baseline; FEV1, forced expiratory volume in 1 second; FOR, formoterol; MCID, minimal clinically important difference; PBO, placebo; QVA149, indacaterol/glycopyrronium; SAL, salmeterol; SGRQ, St George’s Respiratory Questionnaire; TDI, transitional dyspnea index; TIO, tiotropium; UMEC, umeclidinium; VI, vilanterol.