Figures & data
Figure 1 Study design.
Notes:
aIn subsets of patients. bAdditional safety assessments include serial serum potassium, heart rate, blood pressure, and QT interval pre-dose and over 60 minutes post-dose.
Abbreviations: CAT, COPD Assessment Test; EXACT, EXAcerbations of Chronic pulmonary disease Tool; R, randomization; SGRQ-C, St George’s Respiratory Questionnaire for COPD; V, visit; bid, twice a day.
Abbreviations: CAT, COPD Assessment Test; EXACT, EXAcerbations of Chronic pulmonary disease Tool; R, randomization; SGRQ-C, St George’s Respiratory Questionnaire for COPD; V, visit; bid, twice a day.
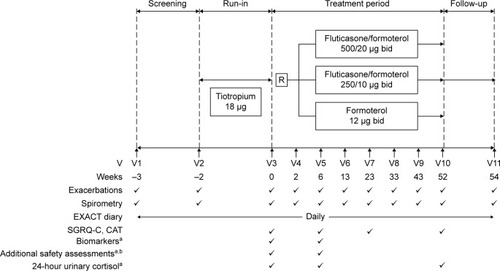
Table 1 Summary of study endpoints
