Figures & data
Table 1 System suitability data of six injections of 2 µg/mL tenofovir and 2 µg/mL ddC as a resolution standard
Figure 2 HPLC chromatograms for (A) blank, (B) system suitability (6 µg/mL tenofovir and 6 µg/mL ddC), (C) EF sample of formulation A, (D) EF sample of formulation B, (E) Caco-2 study sample of formulation A, and (F) Caco-2 study sample of formulation B. Formulations were prepared using 50 mg cholesterol, either 7.5% (formulation A) or 15% (formulation B) stearylamine as a positive charge imparting agent and an amount of phospholipon 100H to make a total lipid pool of 150 mg.
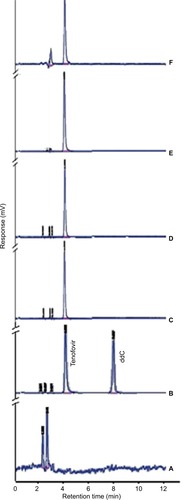
Table 2 Linearity, accuracy, and precision data of tenofovir analysis
Table 3 Robustness data (mean ± relative standard deviation) expressed as nominal, low, and high values for flow rate, injection volume, column temperature, organic-phase fraction, and pH value of mobile-phase variations
Table 4 Entrapment efficiency and apparent permeability (Caco-2 transwells, n = 4) data for tenofovir liposomal formulations