Figures & data
Figure 1 Patient disposition.
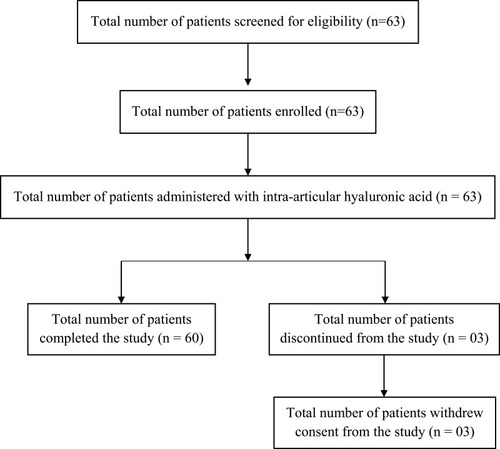
Table 1 Summary of Patient Demographic Characteristics – Safety Population
Table 2 Symptoms of Knee Arthritis Present for Last 3 Months in All Enrolled Patients
Figure 2 Summary of pain KOOS score.
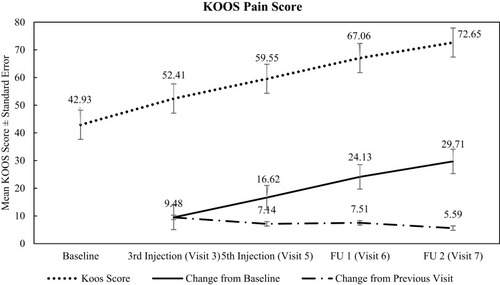
Table 3 Change from Baseline in KOOS Pain Score – PP Population
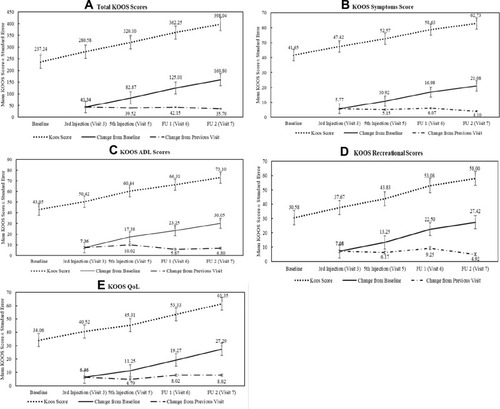
Figure 3 Summary of KOOS score.