Figures & data
Figure 1 Structural formula of arbekacin sulfate.
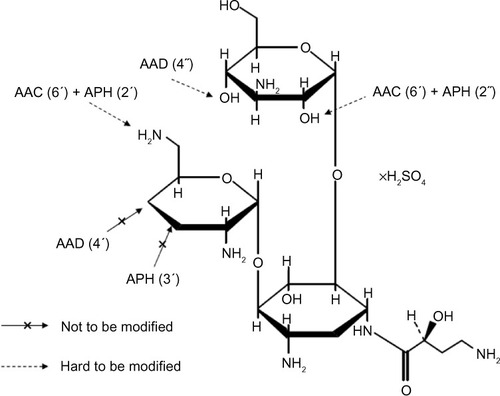
Table 1 In vitro antibacterial activity against aerobic bacteria
Figure 2 Antibacterial activity against MRSA.
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; VCM, vancomycin; TEIC, teicoplanin; ABK, arbekacin; LZD, linezolid; ST, sulfamethoxazole-trimethoprim; RFP, rifampicin; DAP, daptomycin.
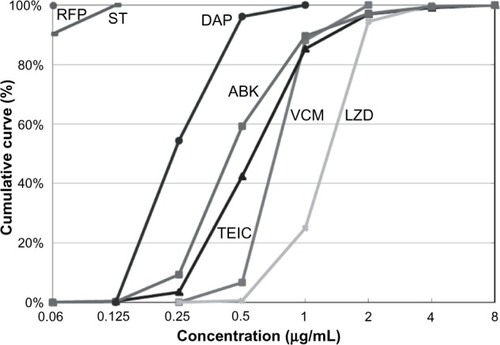
Figure 3 Bactericidal activity of anti-MRSA agents against five MRSA strains.
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; TEIC, teicoplanin; LZD, linezolid; VCM, vancomycin; QPR/DPR, quinupristin/dalfopristin; ABK, arbekacin; SD, standard deviation; CFU, colony forming units.
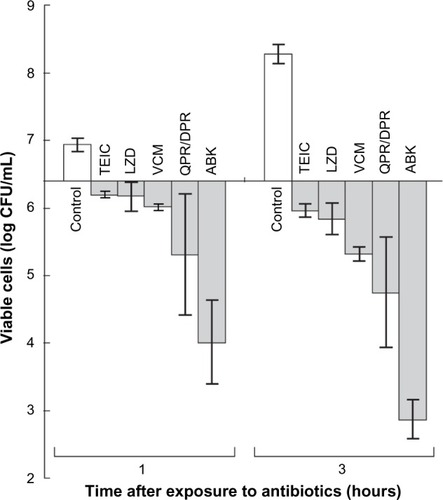
Figure 4 Effect of TSST-1 producing ability of MRSA.
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; ABK, arbekacin; VCM, vancomycin; TEIC, teicoplanin; TSST-1, toxic shock syndrome toxin-1; CFU, colony forming units; MIC, minimal inhibitory concentration; Cmax, maximum concentration.
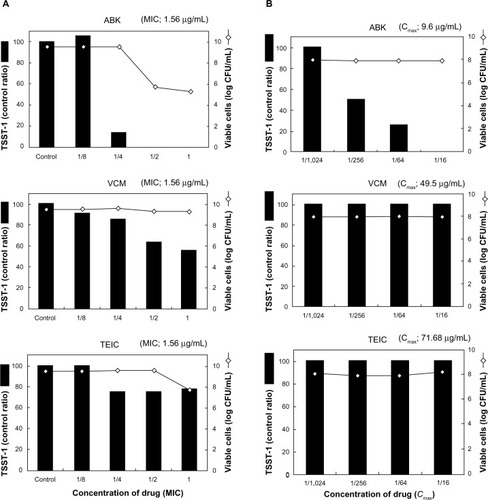
Figure 5 Scoring of combination effect for each drug combination against multidrug-resistant (MDR) Pseudomonas aeruginosa strains.
Abbreviations: MDRP, multidrug-resistant Pseudomonas aeruginosa; AZT, aztreonam; AMK, amikacin; GM, gentamicin; ABK, arbekacin.
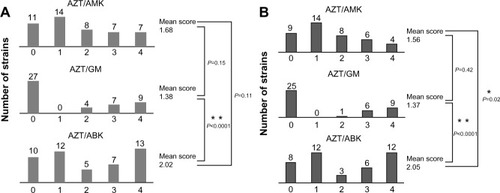
Figure 6 Results of Break-point Checkerboard Plate for (A) colistin plus rifampicin, (B) arbekacin plus aztreonam and (C) amikacin plus aztreonam.
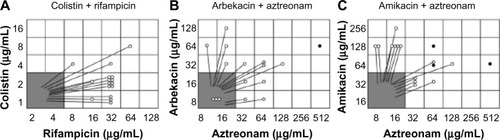
Figure 7 Plasma (serum) concentration after administration of ABK.
Abbreviations: ABK, arbekacin; MRSA, methicillin-resistant Staphylococcus aureus; SD, standard deviation; Ccr, creatinine clearance (mL/min).
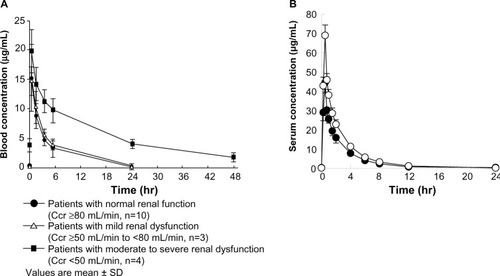
Table 2 Pharmacokinetic parameters after administration of ABK
Table 3 Relationship between final daily dosage and efficacy/adverse drug reaction (ADR) rates
