Figures & data
Figure 1 Study design.
Abbreviations: bid, twice daily; PK, pharmacokinetic.
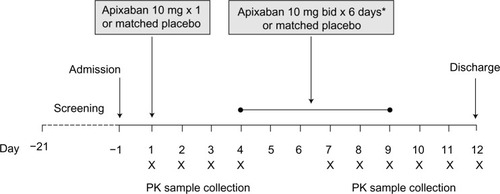
Table 1 Baseline demographics
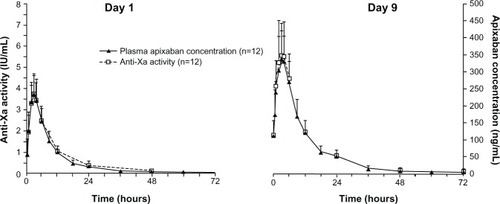
Figure 2 Mean plasma apixaban concentration versus time (n=12) following single-dose administration (day 1) and at steady state (day 9). Error bars show +1 standard deviation from the mean.
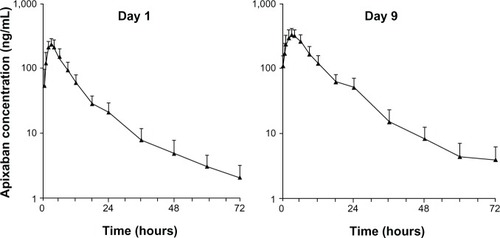
Table 2 Summary statistics of single- and multiple-dose apixaban pharmacokinetic parameters (n=12)