Figures & data
Figure 1 A picture of C-reactive protein (CRP) from 1B09.pdb made using pymol.Citation10 CRP is a pentameric molecule containing a recognition face that binds phosphocholine and calcium ions, and on the opposite side, an effector face that contains a C1q-binding site. Function depends upon Ca2+-dependent ligand binding. See text for details. Reproduced by permission of Skolstoe through Wikipedia commons.
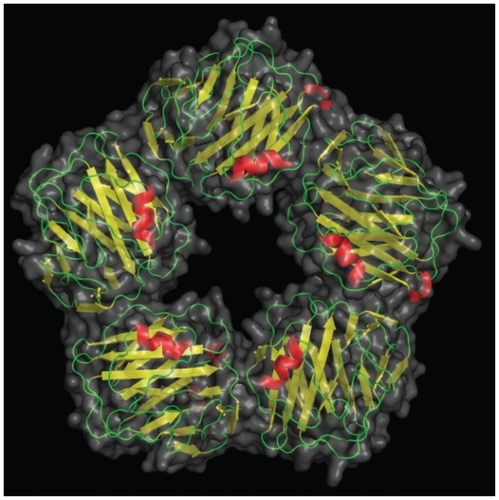
Figure 3 The structure of rosuvastatin, uniquely containing sulfur, a fluorophenyl group, and a modified hydroxyglutaric acid moiety. The IUPAC name of rosuvastatin is (E,3R,5R)-7-[4-(4-fluorophenyl)-2-[methyl(methylsulfonyl)amino]-6-propan- 2-ylpyrimidin-5-yl]-3,5-dihydroxyhept-6-enoic acid.
![Figure 3 The structure of rosuvastatin, uniquely containing sulfur, a fluorophenyl group, and a modified hydroxyglutaric acid moiety. The IUPAC name of rosuvastatin is (E,3R,5R)-7-[4-(4-fluorophenyl)-2-[methyl(methylsulfonyl)amino]-6-propan- 2-ylpyrimidin-5-yl]-3,5-dihydroxyhept-6-enoic acid.](/cms/asset/c5012b3c-b41c-432f-966d-5c007d85b5b8/dddt_a_10812_f0003_b.jpg)
Table 1 Rough equivalent doses of rosuvastatin
Table 2 Baseline clinical data in the JUPITER trial
Table 3 JUPITER trial: comparison of outcomes between treated and nontreated patients
Table 4 Cardiovascular events fell based on LDL cholesterol and on hs-CRP levels <2 mg/L
Table 5 Cardiovascular events fell based on LDL cholesterol and on hs-CRP levels <1 mg/L
Table 6 Event rates and hazard ratios for the primary end point correlated with estimated 10-year Framingham and Reynolds risk scores at baseline
Table 7 Components of the primary end point reached in the JUPITER study
Figure 2 Cholesterol is synthesized via the mevalonate pathway. Acetyl-CoA forms 3-hydroxyl-3-methylglutaryl CoA (HMG-CoA) in several steps. The conversion of 3-hydroxy-3-methylglutaryl (HMG)-CoA to mevalonate, the rate-limiting step in cholesterol synthesis, is catalyzed by HMG-CoA reductase (HMGR), an enzyme within the endoplasmic reticulum. Rosuvastatin is an efficient competitive inhibitor of HMGR, reducing not only mevalonate levels, but also prenylated downstream products. The post-translational process of prenylation is needed for the function of small G proteins, including geranylgeranylation of Rho, Ras, and Rab, necessary for cellular signaling, transduction, and intermembrane translocation. As beneficial as statins are, there are obligatory molecular consequences inherent in their use, some related to their beneficial pleiotropic actions, but also to their side effects. Many steps and enzymes are omitted for clarity.
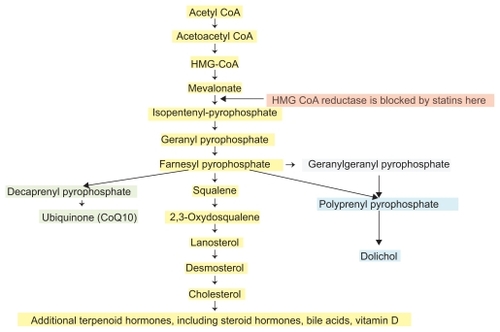
Table 8 Effect of coadministered drugs on rosuvastatin systemic exposure
Table 9 Effect of rosuvastatin coadministration with warfarin, digoxin, and an oral contraceptive