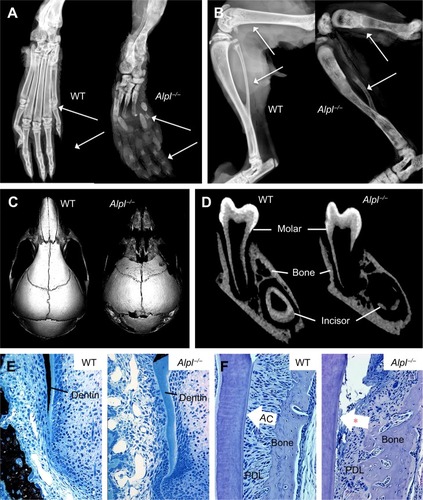
Notes: (
A) Radiographs of hind paws of wild-type (WT) and
Alpl−/− mice at 22 days postnatal (dpn).
Alpl−/− mouse phalanges and metatarsals (white arrows) show gross hypomineralization and deformities. (
B) Radiographs of hind limbs of WT control and
Alpl−/− mice at 22 dpn.
Alpl−/− mouse femurs, tibias, and fibulas (white arrows) exhibit reduced mineralization, bowing, fracturing, and growth plate defects. (
C) Radiographs of skulls of WT and
Alpl−/− mice at 15 dpn.
Alpl−/− mouse cranial bones feature severe hypomineralization and altered craniofacial shape. (
D) Micro-computed tomography (micro-CT) of mandibles of WT and
Alpl−/− mice at 14 dpn.
Alpl−/− mouse molars, incisors, and alveolar bone show radiolucency indicative of severe hypomineralization. (
E) Von Kossa-stained undecalcified tissue sections of WT and
Alpl−/− mouse mandibles at 12 dpn. Compared to well mineralized molar dentin in WT (indicated by black stain),
Alpl−/− mouse molar roots featured hypomineralized dentin matrix (lack of black stain). (
F) H&E tissue sections of WT and
Alpl−/− mouse mandibles at 22 dpn. Compared to the organized and functional periodontal complex in WT,
Alpl−/− mouse molars exhibit lack (*) of acellular cementum (AC), detachment of the periodontal ligament (PDL), and disorganized PDL and alveolar bone. Images in panels
A and
B are reprinted from Bone, Vol /edition 49(2), Yadav MC, Lemire I, Leonard P, et al, Dose response of bone-targeted enzyme replacement for murine hypophosphatasia, Pages 250–256, Copyright (2011), with permission from Elsevier.
Citation79 Images in panel
C are reprinted from Bone, Vol 67, Liu J, Nam HK, Campbell C, Gasque KC, Millán JL, Hatch NE, Tissue-nonspecific alkaline phosphatase deficiency causes abnormal craniofacial bone development in the
Alpl(−/−) mouse model of infantile hypophosphatasia, Pages 81–94, Copyright (2014), with permission from Elsevier.
Citation71 Images in panel
E are reproduced with permission from Copyright © 2013 American Society for Bone and Mineral Research. Foster BL, Nagatomo KJ, Tso HW, et al. Tooth root dentin mineralization defects in a mouse model of hypophosphatasia.
J Bone Miner Res. 2013;28(2):271–282.
Citation70