Figures & data
Table 1 Antibodies for cardiac immature cells (including cardiac stem cells, cardiac progenitors, cardiac precursor cells, and amplifying cells)
Table 2 APS inhibited hyperglycemia and weight loss in diabetes
Table 3 APS protected heart function in diabetes
Figure 1 APS protect cardiac function in diabetes.
Abbreviations: APS, astragalus polysaccharides; APS-DM, diabetic mice with APS treatment; APS-DM-SOD2+/−, diabetic SOD2+/− mice with APS treatment; Ctrl, C57BJ/6J mice were taken as the normal control; DM, diabetic mice; DM-SOD2+/−, diabetic SOD2+/− mice; SOD2+/−, nondiabetic SOD2+/− mice.
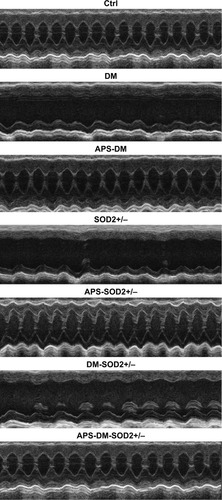
Figure 2 APS increased the abundance of CSPCs in diabetes.
Abbreviations: APS, astragalus polysaccharides; APS-DM, diabetic mice with APS treatment; APS-DM-SOD2+/−, diabetic SOD2+/− mice with APS treatment; Ctrl, C57BJ/6J mice were taken as the normal control; DM, diabetic mice; DM-SOD2+/−, diabetic SOD2+/− mice; IHC, immunohistochemistry; SOD2+/−, nondiabetic SOD2+/− mice.
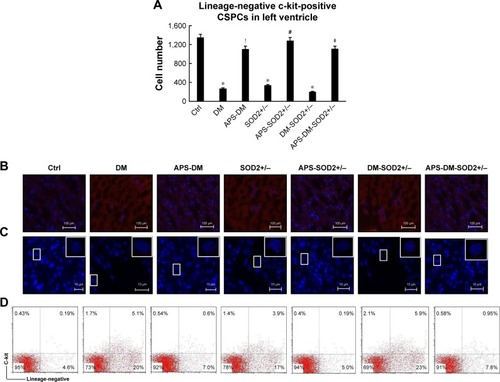
Figure 3 APS inhibited the apoptosis of CSPCs in diabetes.
Abbreviations: APS, astragalus polysaccharides; APS-DM, diabetic mice with APS treatment; APS-DM-SOD2+/−, diabetic SOD2+/− mice with APS treatment; CSPCs, cardiac stem and progenitor cells; Ctrl, C57BJ/6J mice were taken as the normal control; DM, diabetic mice; DM-SOD2+/−, diabetic SOD2+/− mice; SOD2+/−, nondiabetic SOD2+/− mice.
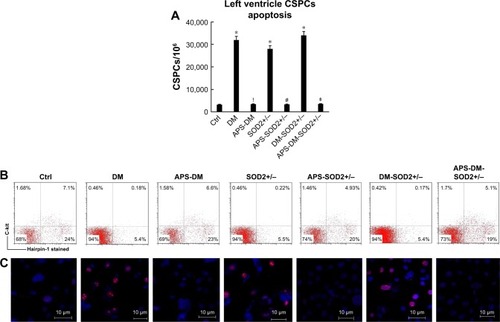
Figure 4 APS enhanced the proliferation of CSPCs in diabetes.
Abbreviations: APS, astragalus polysaccharides; APS-DM, diabetic mice with APS treatment; APS-DM-SOD2+/−, diabetic SOD2+/− mice with APS treatment; CSPCs, cardiac stem and progenitor cells; Ctrl, C57BJ/6J mice were taken as the normal control; DM, diabetic mice; DM-SOD2+/−, diabetic SOD2+/− mice; SOD2+/−, nondiabetic SOD2+/− mice.
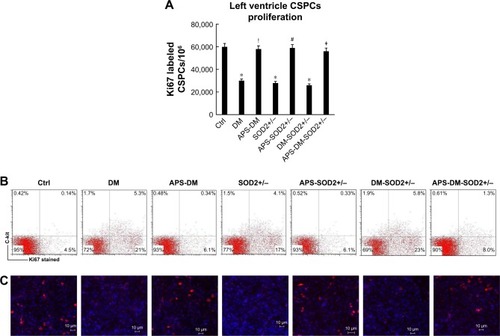
Figure 5 APS inhibited ROS formation and oxidative damage of CSPCs in diabetes.
Abbreviations: APS, astragalus polysaccharides; APS-DM, diabetic mice with APS treatment; APS-DM-SOD2+/−, diabetic SOD2+/− mice with APS treatment; CM-H2DCFDA, 5-(6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate; CSPCs, cardiac stem and progenitor cells; Ctrl, C57BJ/6J mice were taken as the normal control; DM, diabetic mice; DM-SOD2+/−, diabetic SOD2+/− mice; H2O2, hydrogen peroxide; ⋅OH, hydroxyl radicals; ROS, reactive oxygen species; SOD2+/−, nondiabetic SOD2+/− mice.
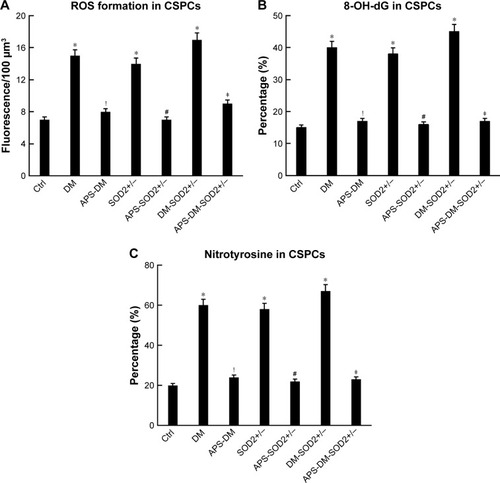
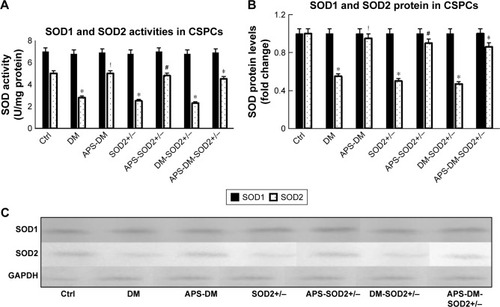
Figure 6 APS enhanced SOD2 enzyme activities and protein levels of CSPCs in diabetes.
Abbreviations: APS, astragalus polysaccharides; APS-DM, diabetic mice with APS treatment; APS-DM-SOD2+/−, diabetic SOD2+/− mice with APS treatment; CSPCs, cardiac stem and progenitor cells; Ctrl, C57BJ/6J mice were taken as the normal control; DM, diabetic mice; DM-SOD2+/−, diabetic SOD2+/− mice; SOD2+/−, nondiabetic SOD2+/− mice.