Figures & data
Figure 1 The two binding sites (G and H) of GSTπ.
Abbreviation: GST, glutathione S-transferase.
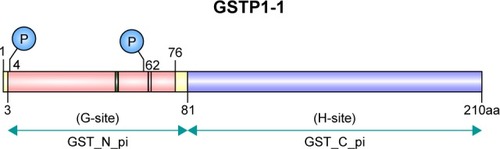
Figure 2 Involvement of GSTπ in the detoxification of exogenous and endogenous substrates.
Abbreviation: GST, glutathione S-transferase.
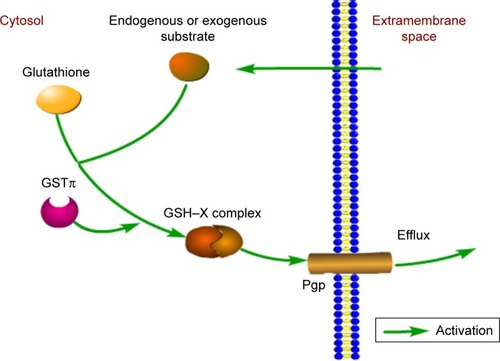
Figure 3 The process of S-glutathionylation.
Abbreviation: GST, glutathione S-transferase.
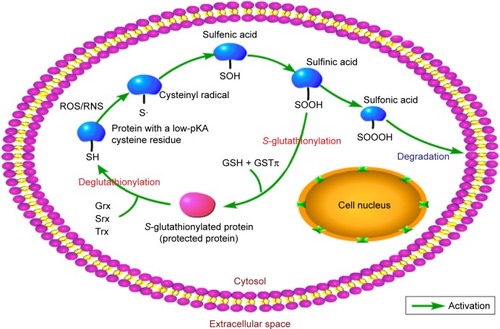
Figure 4 Ligand-binding properties of JNK and TRAF2.
Abbreviation: GST, glutathione S-transferase.
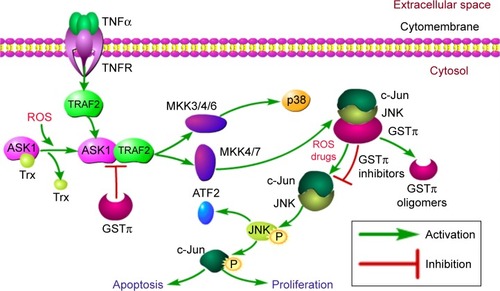
Table 1 Genetic polymorphisms of cytosolic GSTs
Table 2 Antitumor agents targeting GSTπ in context
