Figures & data
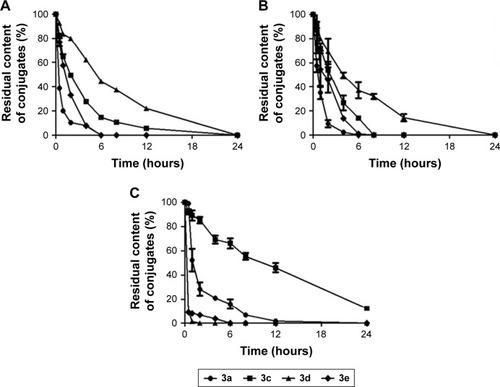
Figure 1 (A) Synthesis of amino acid ester conjugates of budesonide and (B) synthesis of acetates conjugates of budesonide.
Abbreviations: DMAP, 4-dimethylaminopyridine; DMF, N,N-dimethylformamide; EDCI, N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride.
Figure 2 1H NMR spectra (in CDCl3) of Bud conjugates.
Abbreviations: Bud, budesonide; CDCl3, chloroform-d; 1HNMR, proton nuclear magnetic resonance.
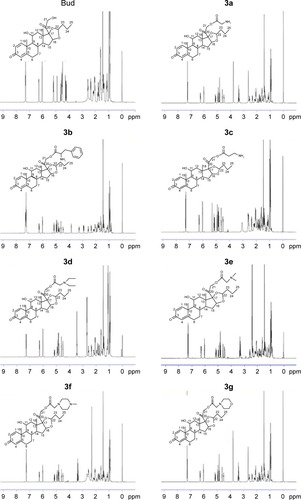
Figure 3 Equilibrium solubility of Bud conjugates at room temperature in distilled water for 72 hours.
Abbreviations: ANOVA, analysis of variance; Bud, budesonide; LSD, least significant difference.
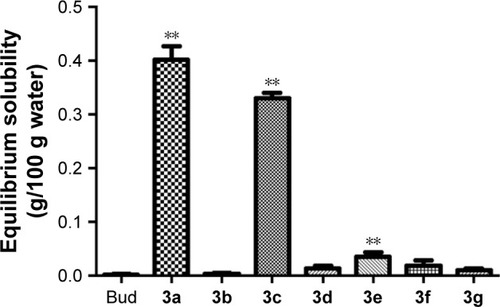
Figure 4 IC5 of Bud conjugates on A549 cell lines.
Abbreviations: Bud, budesonide; IC5, 5% inhibiting concentration.
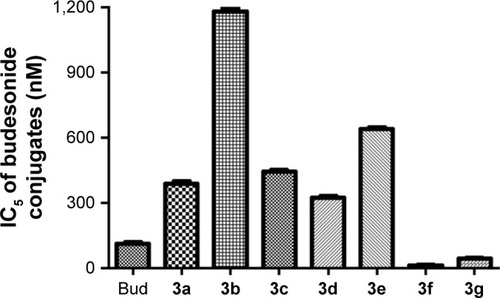
Figure 5 Bud conjugates (1 nM) inhibit the production of IL-6 on LPS-induced A549 cell lines.
Abbreviations: ANOVA, analysis of variance; Bud, budesonide; IL-6, interleukin-6; LPS, lipopolysaccharide; LSD, least significant difference.
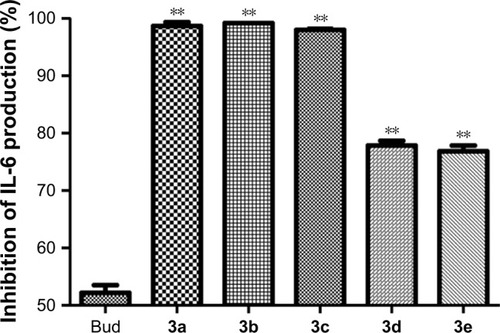
Figure 6 Effects of Bud conjugates on xylene-induced ear edema.
Abbreviations: ANOVA, analysis of variance; Bud, budesonide; DMSO, dimethyl sulfoxide; LSD, least significant difference.
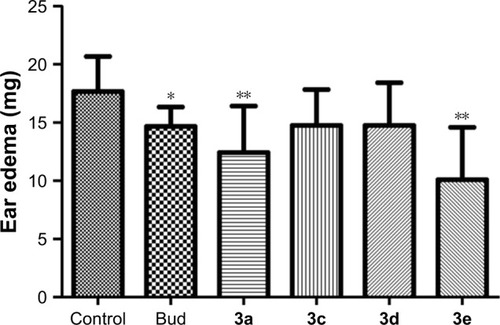
Table 1 Regression equations of budesonide conjugates in different tissue samples for HPLC analysis
Table 2 Recovery and precision for the detection of budesonide conjugates in different tissue samples by HPLC
Figure 7 Hydrolysis behavior of budesonide conjugates in different pH solutions.
Abbreviation: HPLC, high-performance liquid chromatography.
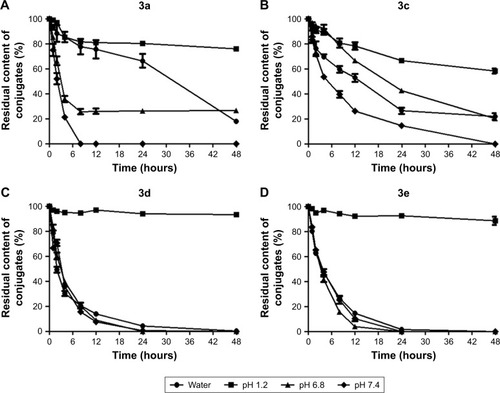
Figure 8 Hydrolysis behavior of budesonide conjugates in rat plasma (A), human plasma (B), and rat lung homogenate (C).
Abbreviation: HPLC, high-performance liquid chromatography.