Figures & data
Table 1 Composition of the emulsions (wt.% = weight percent). F6H8: Perfluorohexyloctane
Table 2 Physicochemical properties of F6H8 emulsions (MDS – mean droplet size (mean± SD); pH; osmolarity)
Figure 1 Apoptosis (A). Influence of F6H8-Pluronic or F6H8-lecithin emulsions or (B) plain surfactant stock solution with either Pluronic or lecithin on Annexin V as marker for apoptosis inperipheral blood mononuclear cells (monocytes) by FACS analysis. Annexin V binding is shown on the x-axis; events/cell counts are shown on the y-axis. Increased levels of apoptosis are visible by the right shift on the x-axis.
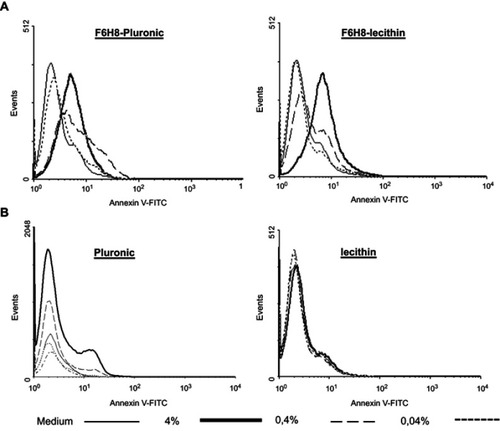
Figure 2 Phagocytosis. Cellular uptake of DiI-F6H8-Pluronic or DiI-F6H8-lecithin emulsions. In these emulsions, F6H8 was fully saturated with the lipophilic DiI and then further processed for emulsification. Peripheral blood mononuclear cell (PBMC) (106 cells/mL) were incubated for 24 hrs with DiI-F6H8-lecithin (panel to the left) or DiI-F6H8-Pluronic (panel to the right) emulsions (4%, w/v). Thereafter, cytospins were prepared and the cells were analyzed by fluorescence microscopy. Original magnification: 400x. White bar: 20 µm. Blue: nucleus of PBMCs; red: phagocytized DiI-F6H8 emulsion droplets.
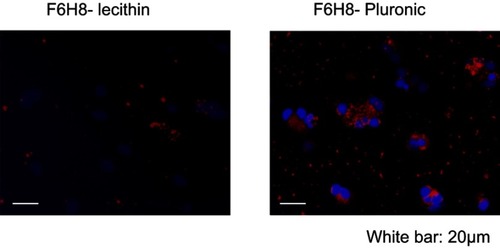
Figure 3 (A) Dose-dependent influence of F6H8-Pluronic or F6H8-lecithin emulsions on TNF-α-mediated expression in whole blood of 5 different donors (*p<0.001 vs control; #p<0.001 vs F6H8-Pluronic). (B) Dose-dependent influence of the two plain surfactant stock solutions of Pluronic or lecithin on TNF-α-mediated expression in whole blood of the same 5 different donors (*p<0.001 vs control; #p<0.001 vs Pluronic).
Figure 4 Anti-oxidative capacity and drug delivery properties of F6H8-cEM. (A) Different dilutions of F6H8-Pluronic (squares) or F6H8-lecithin (circles) emulsions were added to a solution of horseradish peroxidase (5 U/mL), hydrogen peroxide (0.003% w/v), and luminol. The emulsions contained α-tocopherol (+α-toc, open symbols) or not (filled symbols). The stock from which the dilutions were prepared contained 40% (w/v) F6H8, 4% (w/v) Pluronic or lecithin and, if applicable, 775 µg/mL of α-tocopherol. (B) Either F6H8-Pluronic, F6H8-lecithin, F6H8-Pluronic+ α-tocopherol, or F6H8-lecithin+ α-tocopherol emulsions were added to a neutrophil suspension (5.106 cells/mL) containing luminol. Neutrophils were activated by phorbol myristate acetate (PMA; filled bars) or N-Formylmethionyl-leucyl-phenylalanine (fMLP;open bars) for at least 20 mins, during which chemoluminescence was recorded. The results are expressed in relative light units (RLU) of the peak chemoluminescence signal (mean ± SD). All conditions were performed in quadruplicate, and neutrophils from at least 3 different donors were tested.

Figure 5 Renal function after induction of acute kidney injury. Serum creatinine revealed significantly improved renal function in the F6H8-lecithin emulsion group compared to the control group at days 1, 3, and 5 (F6H8-lecithin vs NaCl; *p<0.01; n=6).
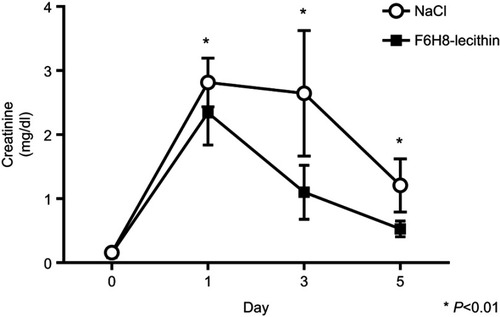
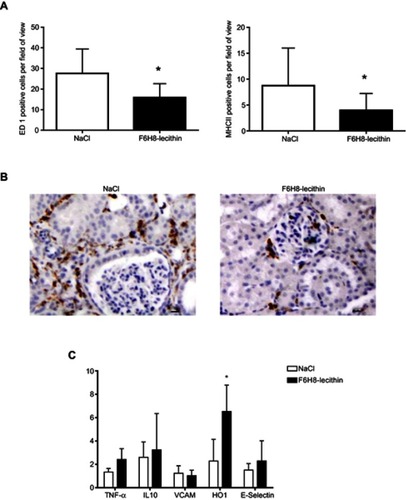
Figure 6 (A) Immunohistology on day 5 revealed significantly less ED1 as well as MHCII positive cells in the renal interstitium of the F6H8-lecithin emulsion treatment group (*p<0.01; n=6 per group). (B) ED1 positive stained cells in renal tissue on day 5 of animals treated with NaCl treated (left panel) and F6H8-lecithin emulsion (right panel) in a rat AKI model. (C) No significant differences were seen in renal tissue harvested 5 days after AKI induction with regard to adhesion molecules (VCAM, E-Selectin) or cytokines (TNFα, IL10) between the groups. The animals treated with F6H8-lecithin emulsion showed significantly elevated expression of HO1 compared to the NaCl control (*p<0.01; n=6 per group).