Figures & data
Figure 1 Structure of human IgG1 antibody and carbohydrate of glycoengineered antibody. (A) The mAbs of human IgG1 isotype consist of two immunoglobulin light chains and two immunoglobulin heavy chains. Heavy chains are covalently paired by disulfide bonds in hinge regions, and each heavy chain is connected to a light chain by a disulfide bond between CH1 and CL. A pair of VH and VL in Fab regions makes an antigen-binding site. In the CH2 domains of Fc regions, an oligosaccharide is covalently attached to the both domains at asparagine 297 (Asn-297). (B) Scheme of the glycoengineered bisected carbohydrate chain of a glycoengineered antibody.
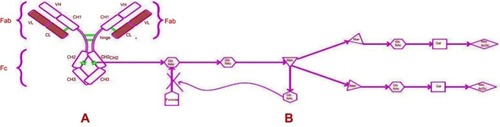
Figure 2 Putative mechanism of action of obinutuzumab.
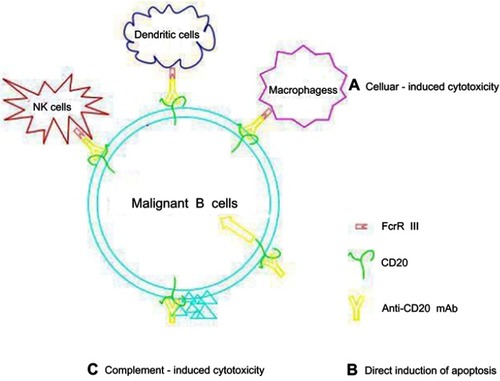
Table 1 Comparison between Type I CD20 mAb and Type II CD20 mAb
Figure 3 Hypothetical model for the 2:1 binding ratio of type I and type II CD20 antibodies binding to CD20 (tetramers, depicted in red). An explanation to explain the 2:1 binding stoichiometry between type I and type II CD20 antibodies is to assume that (A) type I antibodies binding between CD20 tetramer (inter-tetramer, depicted in red) resulting in accumulation in lipid rafts together with FcγRIIb. In contrast, type II (B) antibodies may bind within one tetramer (intra-tetramer).
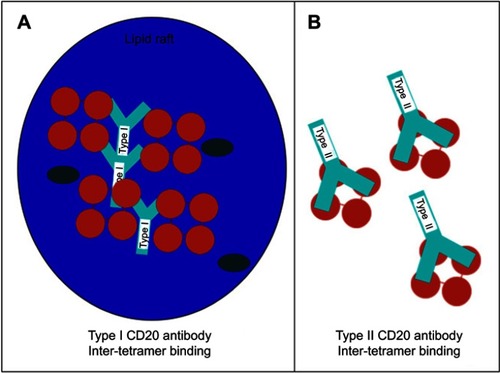
Figure 4 Hypothetical model for CD20 binding of type I and type II CD20 antibodies explaining the impact of FcγRIIb on internalization. (A) Type I antibodies, such as rituximab, may bind to CD20 in a conformation that allows simultaneous binding to FcγRIIb and subsequent cross-linking and activation followed by internalization in lipid rafts. (B) Type II antibodies, such as obinutuzumab, may bind in a conformation that does not allow simultaneous binding to FcγRIIb, thus resulting in low/no internalization.
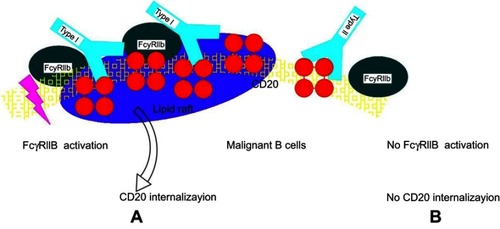
Table 2 Clinical trial of obinutuzumab
Table 3 Clinical trial of combination obinutuzumab with other new agents
