Figures & data
Figure 1 Synthesis of aptamer-conjugated lipid–polymer ligands (PLA-PEG-APT) and redox-sensitive docetaxel prodrug (DTX-GM-TA).
Abbreviations PLA, poly(L-lactide); PEG, poly(ethylene glycol); APT, aptamer; DTX, docetaxel; GM, glyceryl monostearate; TA, thiodiglycolic anhydride; NMR, nuclear magnetic resonance; DTXp, docetaxel prodrug; MAL, maleimide.
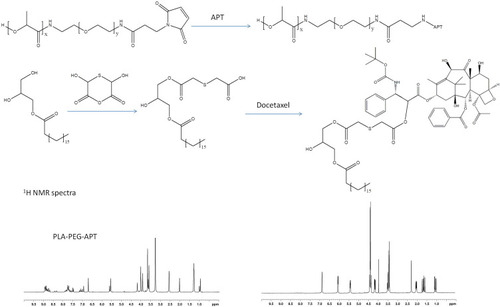
Table 1 Characterization of LPHNs (Mean ± SD, n=3)
Figure 2 TEM images show the morphologies and size of APT-DTXp/DDP-LPHNs and DTXp/DDP-LPHNs.
Abbreviations: TEM, transmission electron microscopy; APT, aptamer; DTXp, docetaxel prodrug; DDP, cisplatin; LPHNs, lipid–polymer hybrid nanoparticles.
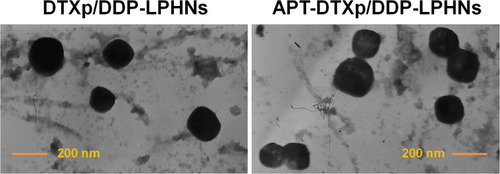
Figure 3 The stability of LPHNs in the presence of serum evaluated by testing the particle size (A), zeta potential (B), and LE (C, D) variations.
Abbreviations: LPHNs, lipid–polymer hybrid nanoparticles; LE, loading efficiency; APT, aptamer; DTXp, docetaxel prodrug; DTX, docetaxel; DDP, cisplatin; FBS, fetal bovine serum.
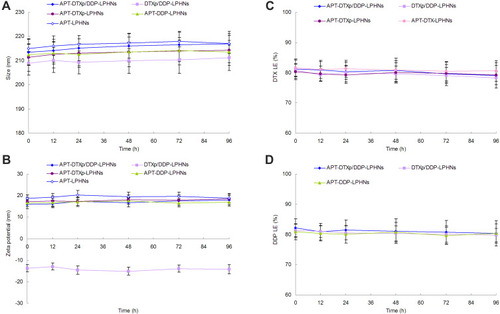
Figure 4 In vitro DTX (A) and DDP (B) release profiles of APT-DTXp/DDP-LPHNs and other LPHNs.
Abbreviations: DTX, docetaxel; DDP, cisplatin; APT, aptamer; DTXp, docetaxel prodrug; LPHNs, lipid–polymer hybrid nanoparticles; H, hypoxic condition; N, normal condition.
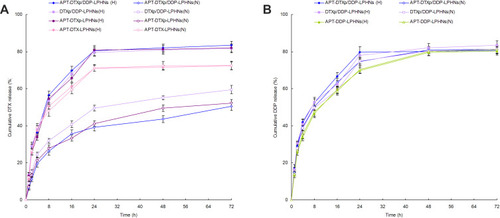
Figure 5 The cell uptake efficiency of APT-DTXp/DDP-LPHNs and DTXp/DDP-LPHNs.
Abbreviations: APT, aptamer; DTXp, docetaxel prodrug; DTX, docetaxel; DDP, cisplatin; LPHNs, lipid–polymer hybrid nanoparticles; FITC, fluorescein 5-isothiocyanate.
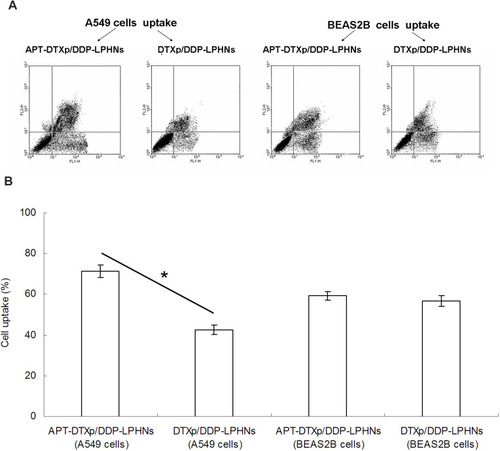
Table 2 CI Values Were Evaluated According to the IC50 Values (Mean ± SD, n=3)
Figure 6 Cell viability of LPHNs evaluated at various drug concentrations using MTT assay.
Abbreviations: LPHNs, lipid–polymer hybrid nanoparticles; MTT, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide; APT, aptamer; DTXp, docetaxel prodrug; DTX, docetaxel; DDP, cisplatin.
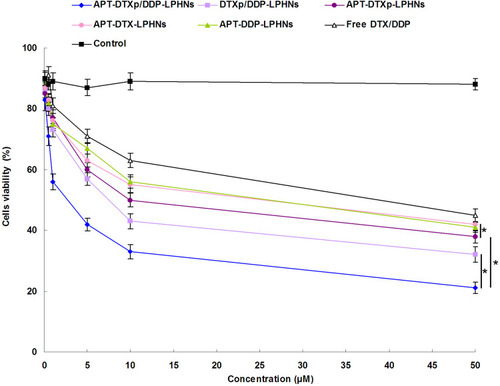
Table 3 BUN, AST, and ALT Levels (Mean ± SD, n=3)
Figure 7 In vivo DTX (A) and DDP (B) biodistribution of drug-loaded LPHNs and free drugs. Note: Free drugs mainly accumulated in kidney, and heart compared with tumor tissue. On the contrary, LPHNs distribution in the tumor was significantly higher than free drugs. APT-DTXp/DDP-LPHNs showed higher tumor distribution than non-decorated DTXp/DDP-LPHNs. Data presented as mean ±SD, n=6. * P < 0.05. DTX: docetaxel; DDP: cisplatin; LPHNs; lipid–polymer hybrid nanoparticles; APT: aptamer; DTXp: docetaxel prodrug.
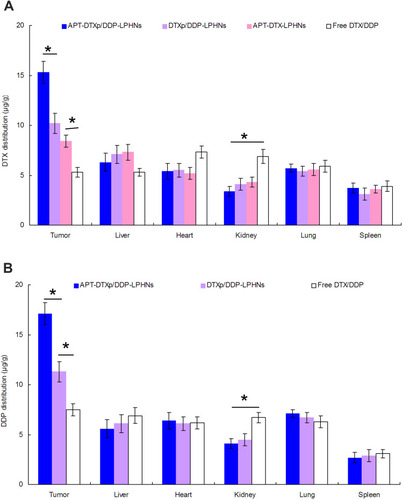
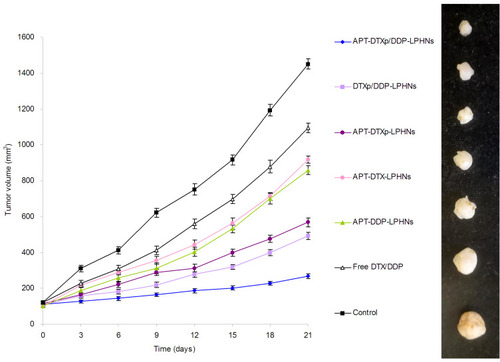
Figure 8 In vivo anti-tumor activity of drug-loaded LPHNs and free drugs.
Abbreviations: LPHNs, lipid–polymer hybrid nanoparticles; DTX, docetaxel; DDP, cisplatin; APT, aptamer; DTXp, docetaxel prodrug.