Figures & data
Figure 1 Mechanisms of diabetic cardiomyopathy. Hyperglycemia and hyperlipidemia induce metabolic changes in the heart that cause mitochondrial dysfunction, oxidative stress, inflammation, and endoplasmic reticulum (ER) stress in cardiomyocytes. Oxidative stress, ER stress, and inflammation can trigger the renin–angiotensin–aldosterone system (RAAS), enhance cardiac sympathetic nerve activity, and calcium-handling dysfunction. These changes mediate cardiac hypertrophy, apoptosis, fibrosis, and microvascular dysfunction, resulting in diastolic and systolic dysfunction.
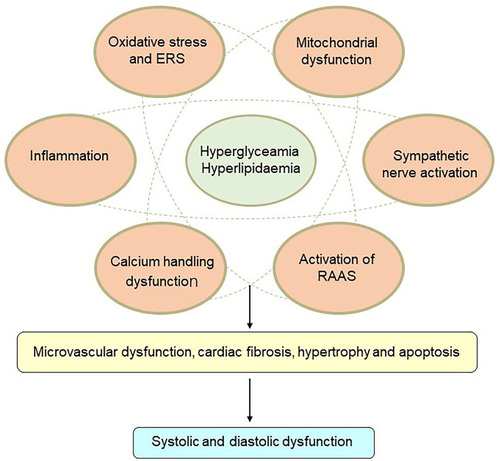
Figure 2 Glucose-lowering mechanisms of SGLT2 inhibitors. The renal proximal tubule accounts for the absorption of all the filtered glucose (~180 g/day) while SGLT2, which is located in the early part of the proximal tubule (S1), accounts for the 80%–90% of filtered glucose reabsorption. Therefore, SGLT2Is prevent major reabsorption (80–90%) of filtered glucose in the early proximal tubule and increase urinary glucose excretion.
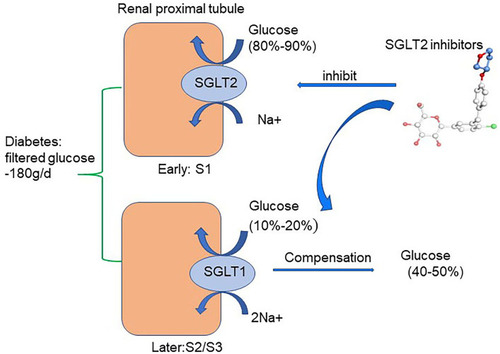
Figure 3 Potential molecular mechanisms of SGLT2 inhibitors on iron homeostasis, mitochondrial function, and cardiac microvasculature in diabetic cardiomyopathy. SGLT2Is can reduce cardiomyocyte sodium (Na+) and calcium (Ca2+) by inhibition of NHE activity in cardiomyocytes. In addition, SGLT2Is also regulate Ca2+ through enhancing SERCA2α function and CaMKII activation. SGLT2Is can enhance mitochondrial function by improvement of mitochondrial fusion–fission proteins, such as Mfn1:Mfn2 ratio and Fis1, as well as Drp1, which is dependent of AMPK activation. Activation of the PGC1α–NRF1–Tfam signaling pathway by SGLT2Is play a crucial role in regulation of mitochondrial biogenesis in the diabetic heart. Microcirculation can be improved by SGLT2Is by eNOS phosphorylation and NO-dependent improvement of endothelial function. All these changes have beneficial effects on attenuation of cardiac stiffness and diastolic dysfunction
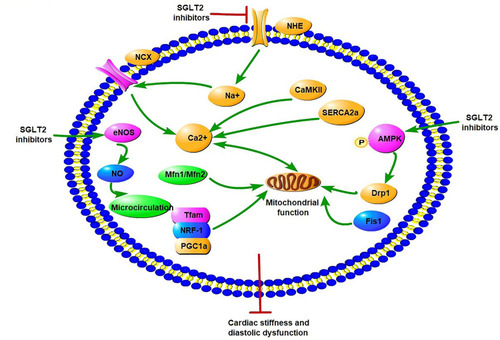
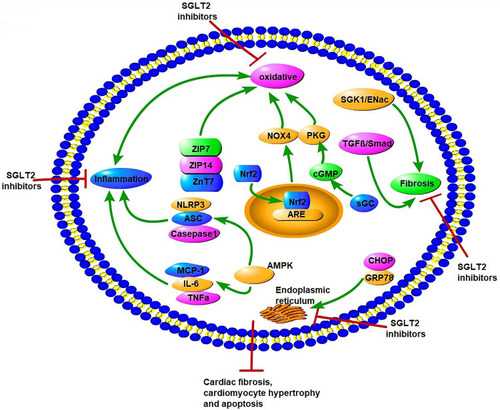
Figure 4 Potential molecular mechanisms of SGLT2 inhibitors on oxidative stress, endoplasmic reticulum stress, inflammation factors, and fibrosis in diabetic cardiomyopathy. SGLT2Is can inhibit oxidative stress by rising levels of free Zn2+ in cardiomyocytes, translocation of Nrf2 to the cell nucleus, activation of the Nrf2–ARE signal, and activation of the sGC–cGMP–PKG pathway. SGLT2Is also inhibit ERS through inhibition of CHOP and GRP78. Inflammation factors, such as NLRP3, ASC, caspase 1, IL6, TNFα, and MCP1, are all attenuated by SGLT2Is dependent of AMPK activation. SGLT2Is prevent cardiac fibrosis through the SGK1–ENac and TGFβ–Smad pathways. All these changes by SGLT2Is lead to attenuation of cardiomyocyte apoptosis, hypertrophy, and fibrosis.