Figures & data
Scheme 1 Organocatalytic synthetic approach to synthesize (S)-2-((S)-2,5-dioxo-1-phenylpyrrolidin-3-yl)-2-methylpentanal (4).
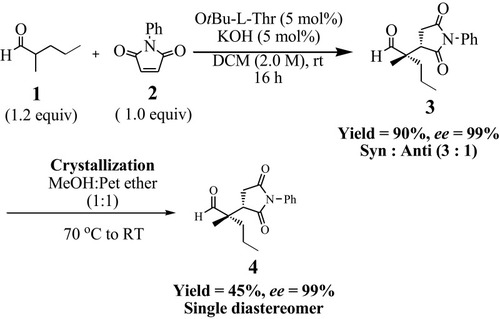
Table 1 In vitro Cyclooxygenase-1/2 and 5-LOX Inhibitions of the Michael Products
Table 2 Animal Group Specification and Quantity of Drug Administered for Acute Toxicity Studies with Synthesized Michael Products
Figure 1 Acetic acid induced writhing results of compounds 4 and 11. The observed values are represented as ±SEM (*P<0.05, **P<0.01, and ***P<0.001 in comparison to the standard).
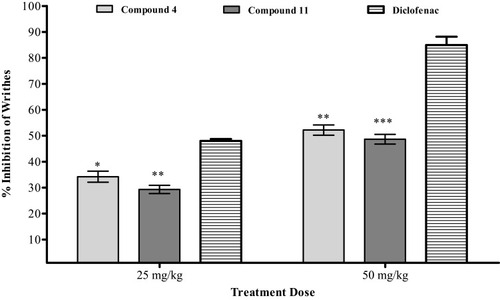
Table 3 Tail Flick Method Results of the Michael Products 4 and 11
Table 4 Formalin-Induced Paw-Licking Response for Elucidation of Central Analgesic Pathway of Compounds 4 and 11
Table 5 Possible Involvement of Alpha-2 Adrenergic Receptor in the Analgesic Response
Table 6 Possible Involvement of Dopaminergic Receptor in the Analgesic Response
Table 7 Interaction with the Amino Acid Residues and Binding Energy Value of the Compounds
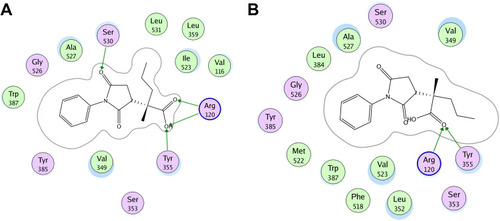
Figure 2 Two-dimensional (2D) interaction plot of compound 4 into the binding site of COX 1 (A) and COX-2 (B). The diagram is generated from MOE software.
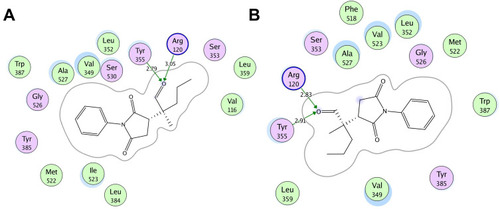
Figure 4 (A) Superposed binding orientation of compounds 9–11 on native ibuprofen (yellow) into the binding site of COX 1. (B–D) Three-dimensional interaction plot of compounds 9–11, respectively, into the binding site of COX-1.
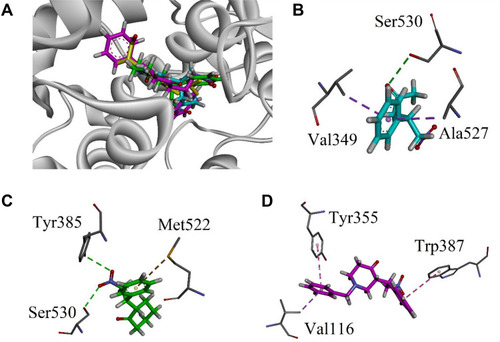
Figure 5 (A) Superposed binding orientation of compounds 9–11 on native SC-558 (yellow) into the binding site of COX 2. (B–D) Three-dimensional interaction plot of compounds 9–11, respectively, into the binding site of COX-2.
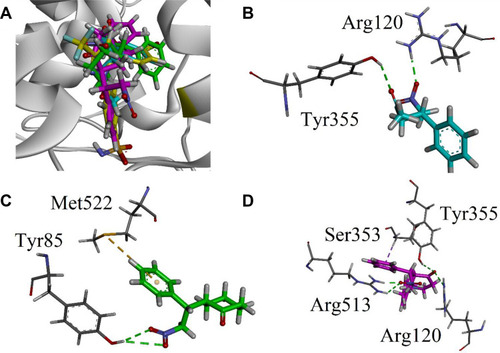
Table 8 Predicted Properties of the Synthesized Compounds (4, 9–11)